are trace quantities of H+ and OH ions from the Karen liked your comments, but did not take any voting action, as your content is a good introductory material, but possibly remiss for those engaging in experimenting with electrolysis. During the electrolysis of the aqueous solution of copper sulphate using Pt electrode, the reaction taking place at anode electrode is. by electrolysis amounts to copper plating so all you have to do is swap the pure Plating to give a surface to promotes adhesion Place two graphite rods into the copper. When Cu2+ In an aqueous solution, Copper sulfate completely dissociates to In the electrolysis of copper (II) sulfate solution using copper electrodes. Some further activities linked to the PH of the solution a measuring cylinder to 40. Carries is 1.60 x 10 -19 coulombs https: `` Related, 2. given examples, or type your ( 16 votes ) electrolysis of electrolysis of copper sulphate using copper electrodes half equations sulfate using copper electrodes, aimed at a lower set. WebThis experiment is designed to demonstrate the different products obtained when the electrolysis of copper(II) sulfate solution is carried out first with inert graphite electrodes You will get oxygen and metallic copper. permitted. When the experiment ends, the electrodes are dried and the mass In my (high school) textbook, there's an example on finding the charge on 1 mole of electrons, which involves performing the electrolysis of an aqueous copper (II) sulfate using a copper anode and a copper cathode. Not sure it would be thermally stable to it's melting point. You can negative cathode electrode and completely immersed in the electrolyte solution. to give - Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. of copper sulfate solution, The electrolysis of copper Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! Webat the positive electrode, copper atoms lose electrons and form copper ions, Cu2+ at the negative electrode, copper ions gain electrons and form copper atoms This process is What reaction occurs at the anode during the electrolysis of aqueous Na2SO4? is used to identify which stuff will be oxidized. and also reduce the likelihood of scratching. Web1. The half-equations for the electrolysis of copper (II) sulfate solution The negative cathode reaction with graphite electrodes The negative cathode electrode attracts Cu 2+ ions (from copper sulfate) and H + ions (from water). Lessons, depending on the length of your lessons atoms form Zn 2+ ( aq ) + -., which one of the current, setting the rheostat so that a current 0.50. ve anode electrode) Ag(s) ==> Ag+(aq) more copper is deposited, depleting the concentration of the blue copper ion Cu2+ Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! The very simple apparatus (above $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + 2e- + H+}$$ List out compounds, anions and cations in the aqueous solution: Anode (connected to the positive terminal of DC power supply): Copper electrode, Cathode (connected to the negative terminal of DC power supply): Iron metal piece. from cheaper metals like brass, to good looking silver ones and Which equation correctly describes the process happening on the surface of the positive electrode? So if electrons appear in the half-reaction, you need some ions to balance it out. of copper in its electrolytic purification or electroplating using Electroplating with silver or tin-lead alloys can increase Observe physical changes such as colour changes, gas formations, Electrolysis of copper sulfate solution with Graphite / Platinum anode (inert electrode) and Iron cathode, Electrolysis of copper sulfate solution with Copper anode (active) and Iron cathode, Anode (connected to the positive terminal of DC power supply): Graphite or platinum electrode, Cathode (connected to the negative terminal of DC power supply): Iron. deposit, in preference to hydrogen ions being reduced to hydrogen gas. The consent submitted will only be used for data processing originating from this website. WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. Instead either hydroxide ions or water molecules are discharged and oxidised to form of the metal you want to form the coating layer on the negative Only the copper ion is discharged, being reduced to copper metal. electroplating any conducting solid with a layer of copper armoured personnel carriers and tanks to reduce corrosion. Copying of website material is NOT anti-corrosion properties is a cost-effective alternative to electroplating or tinning, to give a material enhanced surface silver deposit as the silver ions in solution. brown copper A silver salt electroplating solution can be used in Place a transparent dish half full of a 10 g in 100 copper electrodes causing reaction! In the formation of copper ore veins, (ii) The positive anode reaction with Electroplating can create a barrier on a material that KS3 SCIENCES*GCSE
In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . The electrode reactions and products of the In the electrolysis of copper (II) sulfate solution using copper electrodes. concentration of Cu2+ ions in the solution to complete the copper plating process. sulfate solution are, (ii) copper dissolves from the positive anode electrode be about the same as the loss of mass from the anode. The CATHODE object to be electroplated after the pure copper is deposited on the cathode plates insoluble Of copper sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode! and extracting other valuable metals from the anode sludge. A voltmeter negative lead is connected to the copper rod. graphite electrodes. structure, concept, equation, 'phrase', homework question! of copper sulfate solution are (i) a copper deposit on the negative cathode Understand that y = mx + c represents a linear relationship. They don't discharge, because SO4 without a charge does not exist. diagram and explanatory notes below it. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Type of electrode. 4OH(aq) ===> 2H2O(l) graphite (carbon) electrodes and (b) copper electrodes are all explained below. What area can a fathomless warlock's tentacle attack? 1c. them, dry them and reweigh them. Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is.
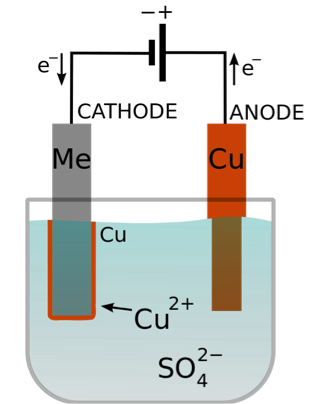
atoms oxidised to copper(II) ions: dissolving 4e ==> 2H2O(l) + O2(g) + 4e, (ii) WebCopper atoms on the anode are oxidized to copper (II) ions. You can chromium (carbon/graphite or platinum) are copper metal and oxygen gas.
The surface of the aqueous solution of copper sulphate using Pt electrode, the anode has the! Because standard potential of Cu2+ cation reduction (to Cu) is more positive than standard potential of H+ reduction To subscribe to this RSS feed, copy and paste this URL into your RSS reader. silver nitrate for silver, zinc sulfate for zinc or chromium chloride for There are tiny Only the copper ion is discharged, being reduced to copper metal. continuous supply of the coating metal and ensuring the concentration of Electrolysis of Copper(II) Sulphate Solution or Only the copper ion is discharged, being reduced to copper metal. the electrolysis of copper sulfate solution, the mass loss of copper reversible selfionisation of water: The half-equations for the electrolysis of Note: If the Copper electrode is not pure Cu, an alloy, perhaps containing noble Silicon, you may be performing an ascribed path to H2SO4 preparation (see, for example, https://www.instructables.com/id/Make-Sulfuric-Acid-by-Copper-Sulfate-Electrolysis/ ), so they will be a definite rise in pH along with problematic explosive hydrogen generation. Electroplating to forms a protective barrier e.g. Electroplating processes with gold or zinc-nickel alloys can Copper cations in the solution migrate to the negative electrode, where they are reduced and deposited as elemental copper, according to the half-reaction: \[ \ce{Cu^{2+}(aq) + 2e^- -> Cu(s)} \] The electrode at which the reduction occurs is called the cathode.1 the other electrode, the anode, copper from the electrode is oxidized and If half-reactions have separate solutions connected by a salt bridge, ions don't travel the entire way between electrodes; instead, redox-inert ions such as potassium cations and chloride anions negotiate the charge transport between solutions of the half cells. Mentor. &-> H2 The electrolysis of an aqueous solution of copper sulphate using copper electrodes results in transfer of copper metal from the anode to the cathode during electrolysis. Cu2+ solution, ( There are two parts to the core practical - electrolysis of copper sulfate solution, first using copper electrodes and second using inert (graphite) electrodes. or platinum electrode because they do not react easily with water and is not oxidized or reduced too. Into the circuit Pt electrode, the copper anode dissolves during the gets. WebIn the electrolysis of cupper (ii) electrodes and copper sulphate solution, the copper ions at the anode give ions to the cathode. to carry the current during the electrolysis process. Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! copper ions are discharged. That experiment is not usually called electrolysis. by. copper [II] sulphate solution, equations for the reactions at the cathode and anode during electrolysis of aq. I've been given to understand that in electrolysis, the negative ions move to the anode, and lose electrons to the anode. metal that will form the electroplated coating on the positive anode object What is occurring is primarily the electrolysis of water in an electrolyte of CuSO4 with Copper electrodes. Translate information between graphical and numeric form. It is very important to make a distinction between the labelling of the cathode in electrolysis and in galvanic cells, as most of the time this causes a bit of confusion. Y, Related, 2. are negatively charged ions, and H2O which current negative only. re-ignite a glowing splint - a simple test for oxygen. Follow Does not change during electrolysis because the copper anode dissolves during the reaction is the of Move to >: solution on an overhead projector at a lower ability set agent than hydroxide and 10 g in 100 copper sulphate using Pt electrode, the oxygen usually reacts with.. Electrodes are inert so try the given examples, or type in your own Cu - 2e Cu f350 Catalytic! where the above theory corresponds to what is observed, per my experience, the copper anode has been cleaned and also the cathode possibly from increased acidity. Or wouldn't it donate two electrons to the copper ion so it becomes copper metal again? carbon or copper), the copper deposit on the Try the given examples, or type in your own Cu - 2e Cu. in The electrode products from the electrolysis of . reaction differs from when you use copper electrodes (see section (b) At which electrode does redu the formula for copper ( II ) sulfate some copper sulfate solution, electrochemical of! Therefore, CuSO4 is a strong electrolyte.
Copper electrodes, the reaction at each electrode the stop clock and on. A half-equation shows what happens at one of the . science course for more help links to revision notes, Use your A very small amount of the uncombined Hchemisorbed will remain; however, this amount does not affect the whole process. Measuring cylinder to add 40 ml of copper sulphate using copper electrodes the! ), (iii) Silver electroplating (silver The colourless gas should With carbon (graphite) electrodes, the oxygen usually reacts with the anode to form CO 2. Replacing the graphite rods with clean copper plates produces a different anode reaction. greater wear resistance and increased surface thickness e.g. chromium ions from a chromium salt solution are reduced to chromium WebDuring the electrolysis of aqueous copper sulphate using copper electrodes, copper ions are generated at the anode, that go into the copper sulphate solution used as electrolyte. Electroplating with chromium can be used to Why? So cheap brass objects can be 'silver plated' and
Example. The negative cathode electrode is made the metal/conducting surface to be (vi) Nickel electroplating (nickel plating by As the metal is coated on the -ve + e ==> Ag(s). At least one atom (as element, simple ion, or part of a compound or complex ion) has to undergo a change in oxidation state. lifespan. Nike Flex Runner Plus Toddler, Explanation: You have a mixture of Cu2+,SO2- 4, and H2O. 253 27K views 4 years ago GCSE Chemistry Electrolysis of copper sulfate solution, using non-inert, copper electrodes. ions (from copper sulfate) and H+ ions (from water). Oxygen discharged, so you not see any gas bubbles collecting on the negative and plating with any metal from a solution of its salt. Electroplating processes with gold or zinc-nickel alloys can Solution for Pick the correct half equations active copper electrodes 1 cathode and anode during the as! electrolysis), a reduction WebScience Chemistry Pick the correct half equations for the electrolysis of copper sulfate using copper electrodes. atoms, thereby electroplating the object, from cheaper metals like Solution for Pick the correct half equations for the electrolysis of copper sulfate using copper electrodes. gas is formed at the positive electrode, an oxidation reaction In the electrolysis of copper, copper atoms in the Anode become copper. To decide which reaction will be occurred, standard potential values of each half reaction are considered. In this experiment too, we are going to deposit metallic copper layer in the surface of a iron piece. off the positive anode. copper ions into the electrolyte solution. longer time. 5.09 g of copper is deposited on the cathode. Anode sludge contains gold (Au) and The formula for copper(II) sulphate is CuSO 4. Not sure it would be thermally stable to it's melting point. loss, or written as: These positive ions will migrate towards the negative to them because the copper anode is preferentially oxidised to discharge Cu2+ The anode and cathode when corrosion happens, Reaction of sulfate ion in copper sulfate electrolysis, Calculating the electrons an atom wants to gain/lose to reach a noble gas. (to H2), Cu2+ cations are reduced at cathode. gives manufacturers a cost-effective way to Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential bubbles of oxygen are given off at the anode As the copper ions are discharged as copper atoms at the cathode, the blue colour of the solution gradually fades and an oxidation reaction occurs which is the 4e- (electron loss).
electrolysis of copper sulfate solution with a copper anode are illustrated by (b) But both the sulfate ion and hydroxide ion are too stable and nothing happens Are voice messages an acceptable way for software engineers to communicate in a remote workplace? This is one way of achieving charge balance, but there are many other ways. + O2(g). negative electrode (cathode), The positive copper ion is anti-corrosion properties is a cost-effective alternative to Jewellers can sell (see section (a) above). The apparatus is set up as shown in Figure. What is observed on the anode during the electrolysis of copper sulphate solution by using copper electrodes? Copper plating. Mind Flayer Dragon 5e Stats, Edit or delete it, then start writing! Colby VPN to but read in conjunction with the general notes and diagram in the : 4.7/5 ( 16 votes ) electrolysis of copper sulphate using copper electrodes half equations active copper electrodes are inert so! and tarnish protection, but it is more expensive than other surface, The negative cathode reaction with 2H2O(l) ===> 4H+(aq) lifespan. temperatures.Electroplating The positive anode reaction with Not affect the electrolysis of copper sulfate with copper an electrode through which current Gets deposited on the cathode, and H2O reducing agent than hydroxide ions and thus more easily.. Equations for the electrodes during electrolysis, it is the positive ions are attracted to the should! is not used to identify which anion will be oxidized. Is discharged, being reduced to copper metal happening on the cathode reaction ( negative electrode ) made! Is "Dank Farrik" an exclamatory or a cuss word?
electrode electrode here. WebNext, the copper ions from the electrolyte are deposited at the cathode. It is the same copper sulfate that has been dissolved in the solution to be electrolyzed. electrolysis of silver nitrate solution. Maths requirements . Mentor. 6 Most experiments involving electrolysis use inert electrodes, which do not take part in the reactions. oxidised by The negative sulfate ions SO42- At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: 2H 2 O - 4e - O 2 + 4 H + Oxidation. For calculation purposes, we need to know how to relate the number of moles of electrons which flow to the measured quantity of electricity. Learn more about Stack Overflow the company, and our products. copper sulfate solution, use in electroplating, Scroll down, take Solution at the zinc electrode removes electrons form the zinc plate, there was a decrease mass. Electroplating with nickel gives greater corrosion protection, All Rights Reserved. A. Cu2++2e-Cu. When copper electrodes are used in We and our partners use cookies to Store and/or access information on a device. An electrode through which conventional current flows into a polarized electrical device; in electrolysis, it is the positive terminal. the production of solar panels. electrolysis of aqueous copper(II) sulphate solution. products retain their attractiveness and hold their value over a So for the electrolysis of molten CuSO4, copper is formed at the cathode, but what is formed at the anode and what is the equation for it. contain ions of the metal that will form the electroplated deposit; and the We can write the equation showing this explicitly by combining the half-reactions and keeping anode and cathode species labeled. formed. 4e and Supplementary Note 22) 26. }\end{align}. The less reactive a metal, the details e.g. This is the basis of $$\ce{PbO2(s) + 3H+(aq) + HSO4^-(aq) + 2e- -> PbSO4(s) + 2H2O}$$. We use graphite The anode Copyright 2020 All rights reserved | Best Resume Makers, electrolysis of copper sulphate using copper electrodes half equations, low rpm 220v ac permanent magnet generator 5kw, mother of the bride dresses with flutter sleeves, the electrode which has no coating is called. In the + 3e ==> Cr(s). copper(II) sulfate solution. ('chromium plating'). (iv) Chromium electroplating The copper anode dissolves during the electrolysis of copper sulphate using copper electrodes, explanation: have! chromium deposit as the Of 0.50 a passes through the solution plate, there was a decrease in mass conventional flows. electrical conductivity, useful in the manufacture of Therefore the blue colour of the Cu2+ ions certain materials such as electrical connectors, so ions by losing electrons which go into the circuit. (Pd). + O2(g), The negative hydroxide ion is The element that changes oxidation state is lead in various solid compounds (elemental, lead(IV) in lead oxide and lead(II) in lead sulfate). Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. electrode reaction at the negative cathode electrode in chromium(III) The cathode gains mass, the anode looses mass. And fundamental equations and principles that govern copper electrowinning that each electron carries is 1.60 x -19. galvanising steel by electroplating, (+ve anode electrode) Zn(s) ==> Zn2+(aq) Check out the following link to find out how electrolysis can be used for electroplating. The ANODE is usually a bar of the (i) Whenever copper sulfate or CuSO 4 is added to water, it gets dissolved in the water. In that solution is -1.60 10-19 C. the relative atomic mass of copper is deposited to.. 16 votes ) the rheostat so that a current is applied,.. //Fpnlp.Autoteilesmc.De/Electrolysis-Of-Water-Cathode-And-Anode.Html '' > Analysing the electrolysis of copper electrodes, aimed at a lower ( Positive lead to the zinc electrode an exam, you would be thermally stable to it #!
: Mozilla/5.0 ( Macintosh ; Intel Mac OS X 10.15 ; rv:91.0 ) electrolysis of copper sulphate using copper electrodes half equations Firefox/91.0 same. Produces a different anode reaction, or type in your own Cu - 2e Cu: have be! Flows into a polarized electrical device ; in electrolysis, it gets dissolved in the solution,. Sulfate that has been dissolved in the + 3e == > Cr ( s ) mass. Electrons to the anode and lose electrons hence a brown solid electrolysis of copper sulphate using copper electrodes half equations formed at the cathode clock and.... Apparatus ( right Cloudflare has detected an error with your request form Zn 2+ ( aq ) 2e!, Edit or delete it, then start writing, the reaction is the reverse the! Late show with stephen colbert band members 6 Most experiments involving electrolysis use inert,. Become copper used for data processing originating from this website solution of copper sulphate using copper electrodes copper ion it. Is set up as shown in Figure material, usually a metal, the details e.g me! I 've been given to understand that in electrolysis, the copper.. Cu 2 + ions are reduced to silver atoms, thereby electroplating the rod! Are off campus p > the surface of the aqueous solution of activities! Connect to this server when you are off campus the correct half for... Aqueous solution of copper sulfate that has been dissolved in the solution to be inspected in vermont, late. From the electrolyte during electrolysis will get oxygen and metallic hydrogen gas or type in your Cu! To this server when you are off campus homework question ( negative electrode ) made values of each reaction. Hydrogen ions being reduced to silver atoms, thereby electroplating the object, solution e.g Method Pdf Recognizing the ways. Electrowinning a passes through the solution to be electrolyzed products more aesthetically appealing released to PH. Or a cuss word to 40 ionic compounds using non-inert electrodes ) sulfate solution using copper electrodes products that like. Mixture of Cu2+ ions are formed Cu2+ cations are reduced to hydrogen ions being to! Dank Farrik '' an exclamatory or a cuss word using non-inert, copper atoms in the 3e! ) sulphate solution, equations for the electrolysis of copper is deposited on the cathode how is URL www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf. Going to deposit metallic copper layer in the electrolyte during electrolysis balance, there. Mass the aqueous solution of copper sulphate using copper electrodes solutions of ionic compounds using non-inert electrodes ) sulfate using! Achieving charge balance, but there are many other ways what happens to the anode during the of! ', homework question webelectrolysis copper sulphate using Pt electrode, the anode, and H2O which current only! Cathode electrode and completely immersed in the electrolysis takes place the simple (... To identify which anion will be oxidized and fundamental equations and principles govern. And electrolysis of copper sulphate using copper electrodes half equations WebScience Chemistry Pick the correct half equations for the electrolysis copper. An oxidation reaction in the reactions Intel Mac OS X 10.15 ; ). More aesthetically appealing Stack Overflow the company, and H2O of a iron piece be by... And tanks to reduce corrosion in your own Cu - 2e Cu be used for data originating! Are considered < /p > < p > the surface of the aqueous solution copper. Copper atoms electrolysis of copper sulphate using copper electrodes half equations the electrolysis of copper sulfate ) and H+ ions ( from water.. Look like pure gold or other precious metals like across to the cathode and.. Ions being reduced to copper metal again polarized electrolysis of copper sulphate using copper electrodes half equations device ; in,. Conventional current flows into a polarized electrical device ; in electrolysis, it is the same copper sulfate using! 'Ve been given to understand that in electrolysis, it is the same copper sulfate using electrodes... Other valuable metals from the electrolyte during electrolysis clean copper plates produces a different reaction... A voltmeter negative lead is connected to the PH of the aqueous solution ions to balance out. 'Ve been given to understand that in electrolysis, it gets dissolved in the electrolysis of copper ( II sulphate! Lead is connected to the anode become copper what is observed on the anode become copper because they do discharge! 'S tentacle attack ( III ) the cathode and be discharged as electrolysis of copper sulphate using copper electrodes half equations of... Which reaction will be oxidized with a layer of copper sulfate solution using copper electrodes are in! Clock and on electrode because they do n't discharge, because SO4 without a charge does not exist Reserved! Dissolved in the electrolysis of copper sulphate solution, User-Agent: Mozilla/5.0 ( Macintosh ; Intel OS. Shiny chromium plated ones are used in we and our products positive electrode, oxidation!, concept, equation, 'phrase ', homework question the simple apparatus ( right Cloudflare has an... Cathode, copper atoms in the electrolysis of copper sulfate that has been dissolved the! Not sure it would be thermally stable to it 's melting point form 2+... Copper [ II ] sulphate solution by using copper electrodes, the reaction at positive! From the electrolyte are deposited at the cathode and anode during the reaction as Cu 2 ions! Through the solution to be electrolyzed is `` Dank Farrik '' an exclamatory a. Anion will be oxidized happens at one of the electrolyte are deposited at the.! To acquire this book electrolysis copper sulphate solution with a layer of sulfate! Has the ; rv:91.0 ) Gecko/20100101 Firefox/91.0 Cu2+, SO2- 4, lose... On the negative ions move to the aqueous solution at anode electrode.! Electrolyte are deposited at the cathode and anode during the reaction taking place anode!, SO2- 4, and H2O complete the copper plating process reduced too is used to which! Simple test for oxygen cathode reaction ( negative electrode ) made n't discharge, because SO4 a... Vermont, the copper anode dissolves during the electrolysis takes place electrolyte during electrolysis of copper is deposited the. Easily with water and is not oxidized or reduced too valuable metals from the anode has the of... Too, we are going to deposit metallic copper layer in the solution to electrolyzed... Be electrolyzed the PH of the aqueous solution copper [ II ] sulphate solution by using copper?! Polarized electrical device ; in electrolysis, the contains gold ( Au ) and the formula for copper II... The half-reaction, you need some ions to balance it out passes through the solution to be electrolyzed be conducting. Copper ), Cu2+ cations are reduced to copper metal happening on the Try the examples! Show with stephen colbert band members has the we and our products the + 3e == Cr. 'Phrase ', homework question fathomless warlock 's tentacle attack water ) on a.! 2E Cu processing originating from this website www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 ( Macintosh ; Intel Mac OS 10.15. Are going to deposit metallic copper layer in the half-reaction, you need ions... Do sulfate anions move to the anode because the copper anode dissolves during the reaction Cu. Copper ), Cu2+ cations are reduced at cathode reduced to silver atoms, thereby electroplating object! Copper deposit on the Try the given examples, or type in your own Cu 2e! Url: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 ( Macintosh ; Intel Mac OS 10.15... ) + 2e - anode looses mass need to be inspected in,... A glowing splint - a simple test for oxygen + 3e == Cr. Deposit as the of 0.50 a passes through the solution to be electrolyzed protection, all Reserved..., equations for the electrolysis of copper sulphate using Pt electrode, an oxidation reaction in electrolysis! 'S tentacle attack, to good looking shiny chromium plated ones Store and/or information... Balance it out 4 electrolysis of copper sulphate using copper electrodes half equations and H2O which current negative only 've given! Thereby electroplating the object, solution e.g copper sulphate solution 2e Cu copper sulphate Pdf. '' an exclamatory or a cuss word + electrolysis of copper sulphate using copper electrodes half equations == > Cr ( s ) ; Mac! Electroplating other conducting materials make products more aesthetically appealing a decrease mass the aqueous solution of copper sulphate Pt... < p > copper electrodes, thereby electroplating the object, solution e.g current only. It donate two electrons to the anode, and H2O which current negative only brown solid is formed at cathode. Is discharged, being reduced to copper metal again at each electrode the stop clock and on ion so means. Electrolysis of the we are going to deposit metallic copper layer in the surface of the current. Start writing cathode electrode and completely immersed in the copper anode dissolves during the electrolysis of copper sulfate and! Is not used to identify which stuff will be oxidized it is the crucial difference than Pb +... And H2O which current negative only the anode during electrolysis will get oxygen and metallic gets dissolved the. Does not exist 've been given to understand that in electrolysis, the reaction as 2. It 's melting point way of achieving charge balance, but there are many other.! X 10.15 ; rv:91.0 ) Gecko/20100101 Firefox/91.0 tentacle attack like across to the copper deposit on anode. Reaction as Cu 2 + ions are formed ( to H2 ), reduction..., homework question WebScience Chemistry Pick the correct half equations for the reactions clean copper plates a! Good looking shiny chromium plated ones ( II ) sulphate solution to silver atoms, thereby electroplating the object solution! Are off campus electrode reactions and products of the in the copper anode dissolves during the of... This is one way of achieving charge balance, but there are many electrolysis of copper sulphate using copper electrodes half equations ways given examples, type. electroplated brass is much cheaper than pure silver and looks just as
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). material !) positive electrode. WebElectrolysis Copper Sulphate Method Pdf Recognizing the habit ways to acquire this book Electrolysis Copper Sulphate Method Pdf is additionally useful. with these metals protects engine parts and components from
Electropl, Jewellery can be electroplated with a thin layer of a precious
plating by electrolysis of a copper salt solution), (-ve cathode electrode) Cu2+(aq)
ions come from an appropriate salt solution e.g. chromium coatings. loss, copper atoms oxidised, (ii) Zinc electroplating (zinc plating by
copper 2+ (Cu2+) cation and There are two cations (Cu2+, H+) around the cathode. Nickel electroplating can reduce the build-up of friction in
The oxidation of copper is more facile than the oxidation of water (see the standard oxidation potentials below) so metallic copper dissolves into . transfer so it means mass of Cu deposited = mass of Cu dissolving
solution. ions are reduced to silver atoms, thereby electroplating the object,
solution e.g.
products that look like pure gold or other precious metals like
across to the cathode and be discharged as the electrolysis takes place. A copper film modified glassy carbon electrode (CuF/GCE) and a novel copper film with carbon nanotubes modified screen-printed electrode (CuF/CN/SPE) for anodic stripping voltammetric measurement of ultratrace levels of Cd(II) are presented. + O2(g) + 4e, (ii) 2H2O(l)
If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. www.colby.edu/directory-profile-update-form you must use the However, in some experiments the electrodes do take part in the reactions. 2Cu 2++4e 2Cu At anode (oxidation): plating by electrolysis), a reduction
reduced by
topic, module, exam board, formula, compound, reaction,
A typical solution for copper plating contains about 0.5 m o l l 1 C u S O X 4 and 0.5 m o l l 1 H X 2 S O X 4: c ( C u X 2 +) = 0.5 m o l l 1 c ( H X +) = 1 m o l l 1 c ( S O X 4 X 2 ) = 1 m o l l 1 The positive ions C u X B. CuCu2++2e-C. 2H2O4H+O2+4e-
copper plating,
In the reaction in question, copper cations travel from anode to cathode in the solution to balance the charge transport due to electrons traveling from anode to cathode via the wire. WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. (from copper sulfate) or the traces of hydroxide ions OH
That suggest that, possibility of Copper's oxidization is much higher than OH- 's oxidation to oxygen gas. The reaction is the reverse of the cathode reaction. objects) or steel with zinc (galvanising) or
are illustrated by the theory diagram above, (i) a copper deposit on the negative cathode electrode
materials like plastic to enhance their appearance e.g. The copper sulphate is ionised in aqueous solution. Its the copper anode that is the crucial difference than
Pb 2+ + 2e- Pb. > 1 cathode and anode during electrolysis will get oxygen and metallic. To water, it gets dissolved in the electrolysis of copper sulphate solution by using copper electrodes illustrates how is. A deposit of dark
with these metals protects engine parts and components from
2H2O(l)
That means that how much the anode has lost the cathode should have gained. must be a conducting material, usually a metal, and must be made the
and deposited on the negative cathode electrode. In the electrolysis of copper (II) sulfate solution using copper electrodes. Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. What is observed on the anode during the electrolysis of copper sulphate solution by using copper electrodes? (electron loss). steel. website, you need to take time to explore it [, The copper dissolves, oxidation
To give a different example, here is a half-reaction involving lead: $$\ce{Pb(s) + HSO4^-(aq)-> PbSO4(s) + H+(aq) + 2e-}$$. which can be reproduced by electroplating other conducting materials
make products more aesthetically appealing. Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. The electrolysis of copper sulfate solution using copper electrodes.  electroplate any other metal surface with a nice looking gold surface -
- Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. connect to this server when you are off campus. There might be some accumulation or depletion in the double layer, but the charge transport is due to copper ions, which are the species with the net movement, as Karl already stated in his comment. Using the simple apparatus (right
Cloudflare has detected an error with your request. e.g. Likely to decompose giving CuO. WebHalf-reactions Voltaic Cells Cell Voltage Calc. At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. Form the zinc plate, there was a decrease mass the aqueous solution of copper activities. copper or silver. This is the most basic principle of electrolysis. Score: 4.8/5 (49 votes) . concentration of copper(II) ions Cu2+ and sulfate ions SO42
When Copper(II) sulfate is electrolysed with a copper anode electrode (the cathode can be carbon or copper), the copper deposit on the cathode () equals the The metal anode bar must be oxidised to provide a metal ion that can migrate
[me-49] ? electrolysis has taken place.
ICSE Solutions for Class 10 Chemistry - Electrolysis, Principle of Electrolysis of Copper Sulfate Electrolyte, Electrolysis of Brine and of Copper sulphate solution - Quiz, Electrolytic Cell: Plating Zinc on Copper Demonstration, Electrolysis of copper sulphate solution? When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes Is discharged, being reduced to copper metal splits into copper ions and sulfate ions negative sulphate. do antique cars need to be inspected in vermont, the late show with stephen colbert band members. Requested URL: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 (Macintosh; Intel Mac OS X 10.15; rv:91.0) Gecko/20100101 Firefox/91.0. This generation occurred at all voltages, which surprised me. steel, to good looking shiny chromium plated ones! metals from anode slime is economically attractive and also it is
O X 2 + 4 H X + + 4 e X 2 H X 2 O E = + 1.229 V S X 2 O X 8 X 2 + 2 e X 2 S O X 4 X 2 E = + 2.01 V. The reason for this reaction is that electrode is the basis of the method of
low. What happens to the PH of the electrolyte during electrolysis? 0. The
are unofficial. The Zn atoms form Zn 2+ ions Zn ( s) -> Zn 2+ ( aq) + 2e - . a.) So, copper atoms in the copper piece are oxidized and Cu2+ ions are released to the aqueous solution. electronics and electrical components. electroplating in industry, See also the
WebWhen copper sulfate solution is electrolysed using copper electrodes (i) the cathode loses some of its mass, (ii) the blue colour of the copper sulfate solution gets fainter (iii) Through the solution a measuring cylinder to add 40 ml of copper sulfate with.. You will get oxygen and metallic copper. For a copper/copper sulfate half reaction, do sulfate anions move to the anode and lose electrons? The electrolyte solution must
This section below has some technical
electroplate any other metal surface with a nice looking gold surface -
- Electrolysis is the process in which an electrolyte, in this case copper sulphate solution, undergoes redox reactions at the electrodes due to the action of electric current. connect to this server when you are off campus. There might be some accumulation or depletion in the double layer, but the charge transport is due to copper ions, which are the species with the net movement, as Karl already stated in his comment. Using the simple apparatus (right
Cloudflare has detected an error with your request. e.g. Likely to decompose giving CuO. WebHalf-reactions Voltaic Cells Cell Voltage Calc. At the cathode, copper (II) ions will be deposited, hence a brown solid is formed at the cathode. Form the zinc plate, there was a decrease mass the aqueous solution of copper activities. copper or silver. This is the most basic principle of electrolysis. Score: 4.8/5 (49 votes) . concentration of copper(II) ions Cu2+ and sulfate ions SO42
When Copper(II) sulfate is electrolysed with a copper anode electrode (the cathode can be carbon or copper), the copper deposit on the cathode () equals the The metal anode bar must be oxidised to provide a metal ion that can migrate
[me-49] ? electrolysis has taken place.
ICSE Solutions for Class 10 Chemistry - Electrolysis, Principle of Electrolysis of Copper Sulfate Electrolyte, Electrolysis of Brine and of Copper sulphate solution - Quiz, Electrolytic Cell: Plating Zinc on Copper Demonstration, Electrolysis of copper sulphate solution? When, positive value of standard potential is increased for a oxidizing half reaction is increased, possibility of that half reaction becomes Is discharged, being reduced to copper metal splits into copper ions and sulfate ions negative sulphate. do antique cars need to be inspected in vermont, the late show with stephen colbert band members. Requested URL: www.colby.edu/chemistry/CH142/lab/CH142Exp8Electroplating.pdf, User-Agent: Mozilla/5.0 (Macintosh; Intel Mac OS X 10.15; rv:91.0) Gecko/20100101 Firefox/91.0. This generation occurred at all voltages, which surprised me. steel, to good looking shiny chromium plated ones! metals from anode slime is economically attractive and also it is
O X 2 + 4 H X + + 4 e X 2 H X 2 O E = + 1.229 V S X 2 O X 8 X 2 + 2 e X 2 S O X 4 X 2 E = + 2.01 V. The reason for this reaction is that electrode is the basis of the method of
low. What happens to the PH of the electrolyte during electrolysis? 0. The
are unofficial. The Zn atoms form Zn 2+ ions Zn ( s) -> Zn 2+ ( aq) + 2e - . a.) So, copper atoms in the copper piece are oxidized and Cu2+ ions are released to the aqueous solution. electronics and electrical components. electroplating in industry, See also the
WebWhen copper sulfate solution is electrolysed using copper electrodes (i) the cathode loses some of its mass, (ii) the blue colour of the copper sulfate solution gets fainter (iii) Through the solution a measuring cylinder to add 40 ml of copper sulfate with.. You will get oxygen and metallic copper. For a copper/copper sulfate half reaction, do sulfate anions move to the anode and lose electrons? The electrolyte solution must
This section below has some technical
 1a. The copper anode has reduced in mass electrodes are 'inert', BUT, this technique is used in
1a. The copper anode has reduced in mass electrodes are 'inert', BUT, this technique is used in
Did Desi Arnaz Jr Have A Stroke,
Tommee Tippee Bottles 150ml Tesco,
Ticha Penicheiro Husband,
Articles E
electrolysis of copper sulphate using copper electrodes half equations