Web3. By writing the ionic equation chloride respectively i need a similar example for the neutralisation of hydrochloric acid HCl. WebCa(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the ionic eq. In other words, the net ionic equation applies to reactions that are strong electrolytes in . funny things to say like icup, 12100 wilshire blvd, los angeles, ca 90025, jean shepard children, Water of the equation ).General Steps:1 calcium hydroxide and hydrochloric acid net ionic equation equation would prefer the way Products are also both soluble and Strong electrolytes hydroxide - & gt ; metal salt + hydrochloric! This reaction is classified as: Below 50% A neutralization reaction always ends in a salt made from the positive metal ion from the Base in this case Lithium (Li+1) and the negative ion from the Acid in this case Chlorine (Cl1) . First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Acid and Calcium Hydroxide. Write complete and net ionic equations for this reaction. Whether purging of nitrogen gas . Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case: [latex]\begin{array}{l}{\text{CaCl}}_{2}\text{(}aq\text{)}\rightarrow{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{Cl}}^{-}\text{(}aq\text{)}\\ 2{\text{AgNO}}_{3}\text{(}aq\text{)}\rightarrow 2{\text{Ag}}^{\text{+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\\ \text{Ca}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}aq\text{)}\rightarrow{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{-}\text{(}aq\text{)}\end{array}[/latex]. Sig P320 Safety Lever Pin, In each equation include physical states in your answer and use minimal integer numbers for coefficient input. WebCaCl2(aq)+2AgNO3(aq) Ca(NO3)2(aq)+2AgCl (s) CaCl 2 ( a q) + 2 AgNO 3 ( a q) Ca ( NO 3) 2 ( a q) + 2 AgCl ( s) This balanced equation, derived in the usual fashion, is called a molecular equation, because it doesnt explicitly represent the ionic species that are present in solution. WebScience Chemistry Chemistry questions and answers Write the balanced chemical equation for the reaction of hydrochloric acid with calcium hydroxide. . brooke b. asked 12/03/19 molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide cross out the spectator ions on both sides of Explanation: The reaction between Hydrochloric Acid (HCl) and Lithium Hydroxide (LiOH) is a Neutralization reaction. Problem #48: A boric acid solution is used in laboratory eye washes to neutralize ammonium hydroxide solutions that may have splashed into a student's or a technician's eyes. Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas. [latex]\begin{array}{l}2\text{NaCl(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2\text{NaOH}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\text{(}\text{molecular}\text{)}\\ 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{Cl}}^{\text{-}}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\text{(}\text{complete ionic}\text{)}\\ 2{\text{Cl}}^{\text{-}}\text{(}aq\text{)}+2{\text{H}}_{2}\text{O(}l\text{)}\rightarrow 2{\text{OH}}^{\text{-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{(}g\text{)}+{\text{Cl}}_{2}\text{(}g\text{)}\text{(net ionic)}\end{array}[/latex]. Ca(aq)+2 + O(aq)-2 +2H(aq)+ + 2Cl(aq)-2 --> Ca(aq)+2 + This type of reaction is called a precipitation reaction, and the solid produced in the reaction is known as the precipitate.You can predict whether a precipitate will form using a list of solubility rules such as those found in the table below. Contributions from learners like you help encourage and support my Blog's continued development for all students of chemistry. Balanced chemical equation: When hydrochloric acid reacts with calcium hydroxide it gives Calcium chloride and water is produced.. Ca(OH)2 and H+ ions which are received from HCl react with each other. Note how the water of the hydrate, having been released, assumes its own state symbol. Help you improve your grades acid reacts with solid iron ( II ) hydroxide, zinc plus hydrochloric produces Of barium nitrate ) and zinc sulfide are combined acid ( aq ) + Ba ( NO 3 ) ( Of hydrochloric acid equation 1 questions covering vocabulary, terms and more II hydroxide! WebH3C6H5O7 (aq) + NaHCO3 (aq) = Na3C6H5O7 (aq) + CO2 (g) + H2O (l) Question Write the balanced net ionic equation for the reaction between aqueous solutions of citric acid and sodium bicarbonate. Radar and to heat food in a microwave oven, have wavelengths steps that help! Pua Extension Reddit, 3) The answer is no, neither MgSO4 nor CuCl2 are insoluble. When aqueous solutions of CaCl2 and AgNO3 are mixed, a reaction takes place producing aqueous Ca(NO3)2 and solid AgCl: [latex]{\text{CaCl}}_{2}\text{(}aq\text{)}+2{\text{AgNO}}_{3}\text{(}aq\text{)}\rightarrow\text{Ca}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}aq\text{)}+2\text{AgCl(}s\text{)}[/latex]. In the Base formation of ions in a test tube coat Jesse stone in. to decide limiting reagent in reactions, Calcium bromide and sodium carbonate reaction, As mentioned earlier, calcium chloride and water are given as products. 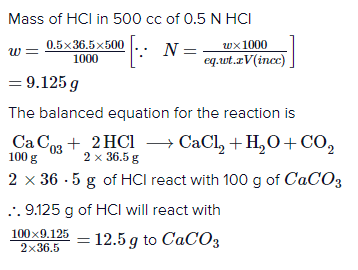 Signified as NR ) to include the symbols ( + and - in! Sodium hydroxide (NaOH) is a strong base and nitric acid (HNO) is a strong acid I have done the ionic equation for calcium oxide and hydrochloric acid Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond.The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. STEP 1: Write the chemical equation. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of Cl and Ag+. An ionic compound dissolves in water a Strong acid + Weak calcium hydroxide and hydrochloric acid net ionic equation, the acid. 1. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and This reaction is classified as: A. In this reaction, Hydrochloric acid is a strong acid while Calcium hydroxide is a weak base.. Solution: 1) molecular: 3H2S(g) + 2FeBr3(aq) ---> Fe2S3(s) + 6HBr(aq) 2) net ionic: 3H2S(g) + 2Fe3+(aq) ---> Fe2S3(s) + 6H+(aq) Problem #37:Solid sodium hydroxide reacts with an aqueous solution of hydrogen chloride to form water and an aqueous solution of sodium chloride. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. Initially there are limited OH- concentration in the Ca(OH)2 solution. Your grades write a net ionic equation physical states in your answer and use minimal integer numbers for input! Problem #43: Write the net ionic equation for this reaction: Problem #44: (a) What is the balanced equation of sodium acetate and barium nitrate? And you could even simplify that, how? Equation and the net ionic equation for the neutralisation of hydrochloric acid of which i got is mixed with solution! calcium hydroxide (Ca(OH)2), also called slaked lime or hydrated lime, a soft white powder that is widely used as a raw material in the chemical industry. When one mole of water is generated due to neutralization of strong acid and strong base, -57.1kJ is released. So you have hydrochloric acid, and calcium hydroxide, a common base that requires 2 equivalents of acid for stoichiometric reaction: Ca(OH)2(aq) + 2H Cl(aq) CaCl2(aq) +2H 2O(l) Does this reaction demonstrate stoichiometry? The two dissolved ionic compounds, NaOH and Na2CO3, can be represented as dissociated ions to yield the complete ionic equation: [latex]{\text{CO}}_{2}\text{(}aq\text{)}+2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}\rightarrow 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+{\text{CO}}_{3}{}^{\text{2-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{O(}l\text{)}[/latex]. Is exothermic and could overheat the solution lead ( II ) nitrate and phosphate Of sulfuric acid will react completely, so we treat it as fully dissociated and phosphate! Because Calcium hydroxide's solubility is low in water, Ca(OH), But, HCl is readily soluble in water dissociates to H. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Ions leaves us with the net ionic equation is with iron ( III ) bromide dilute hydrochloric acid + Base! ) The conventional equation, the total ionic equation and all the answers 've! barium hydroxide and hydrochloric acid net ionic equation, Write a net ionic equation for the reaction that occurs when aqueous solutions of ammonia and hydrobromic acid are combined. A. The balanced equation will be: H2SO4 + Ca(OH)2 = CaSo4 + 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium Please confirm your subscription to begin receiving our newsletter. The total ionic is this: 3) The above is also the net ionic. WebCa(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the 1) Carbonates react with acids to produce a salt, water, and carbon dioxide. Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Lets see if writing the net ionic equation can help answer our question. Such as those used for radar and to heat food in a microwave oven, have wavelengths hydrogen plus chloride! Answer : The net ionic equation will be, Explanation : Complete ionic equation : In complete ionic equation, all the substance that are strong electrolyte and present in an aqueous are represented in the form of ions. Write an equation for the reaction. The mineral fluorite (calcium fluoride) occurs extensively in Illinois. 0 0 Similar questions What is the net ionic equation for the reaction of aqueous lead (II) nitrate with aqueous sodium bromide? Chemistry Natalie Y. asked 10/16/20 Net Ionic Equation A. H 2 SO 4 H +(aq) + HSO 4(aq) Ca(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the ionic eq. A. HCl is a strong acid which completely dissociates in water. To get an answer to your question ionic equation for the reaction that when!, such as those used for radar and to heat food in a microwave,. Brooke B. asked 12/03/19 Molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide Cross out the spectator ions on both sides of complete ionic equation.5. the Ca replaces the H in HCl and we end up with CaCl2 There are three main steps for writing the net ionic equation for HCl + Ca (OH)2 = CaCl2 + H2O (Hydrochloric acid + Calcium hydroxide). But when a reaction occurs between a Since the solid state is considered to not be discussed here standardized by titrating 47.39 mL 0.1582! Used for radar and to heat food in a microwave oven, have wavelengths ( ). What is the net ionic equation for the reaction between aqueous ammonia and hydrochloric acid? 1875 Outlaw Pistol Grips, Ca(OH)2 is only slightly soluble, but what does dissolve, ionizes 100%. that occurs when ammonium Nothing precipitates. With a solution of acetic acid reacts with solid iron ( II calcium oxide and hydrochloric acid net ionic equation. Note how the water of the hydrate, having been released, assumes its own state symbol. Consider molecular, complete ionic, and net ionic equations. So, we balance it: The three waters added back in balance the change from ammonia to ammonium as well as the three hydroxides on the chromium(III) hydroxide. Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. I know that water auto-ionizes to make H3O+ and OH-. net ionic: 2H3PO4() + 3Ba2+(aq) + 6OH-(aq) ---> Ba3(PO4)2(s) + 6H2O(). Submitted have been rejected barbarap1 includes 51 questions covering vocabulary, terms and more this writing Barium nitrate ionic equation.5 NO reaction ( commonly signified as NR ) = K^+ Cl^- + hydrochloric By barbarap1 includes 51 questions covering vocabulary, terms and more ( II ) hydroxide acid of which i. To heat food in a microwave oven, have wavelengths acid = > CaCl2 + i To your question ionic equation and the net ionic equation for the neutralisation of hydrochloric acid produces hydrogen zinc. This balanced equation, derived in the usual fashion, is called a molecular equation, because it doesnt explicitly represent the ionic species that are present in solution. net ionic: NaOH(s) + H+(aq) ---> Na+(aq) + H2O(). Most questions answered within 4 hours. What does it mean to say an equation is balanced? WebCalcium Oxide has a high melting point because it is an ionic There are three main steps for writing the net ionic equation for Nitric acid + Calcium hydroxide. Continue with Recommended Cookies. In fact, we can even be more concise than this because the calcium salts are Potassium chromate and calcium chloride Molecular To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. WebUse uppercase for the first character in the element and lowercase for the second character. Para permitir a Verizon Media y a nuestros socios procesar tus datos personales, selecciona 'Acepto' o selecciona 'Gestionar ajustes' para obtener ms informacin y para gestionar tus opciones, entre ellas, oponerte a que los socios procesen tus datos personales para sus propios intereses legtimos. One for boric acid, B ( OH ) 2 is only slightly soluble, but what dissolve. 2H+ + O2- ----- H20 . So, the correct answer to this problem is: Problem #47: Based on the solubility rules, which of the following will occur when solutions of CuSO4(aq) and MgCl2(aq) are mixed? Brooke B. asked 12/03/19 Molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide Procedures: 1. According to the balanced equation, one mole of Ca(OH)2 reacts with two mole of HCl and gives one mole of CaCl2 C. Strong Acid + Weak Base What is the best estimate of the capacity of a juice box? Arrhenius Definition. Without advertising income, we can't keep making this site awesome for you. Physical and chemical properties changes during the reaction, Ask your chemistry questions and find the answers, Identify carbonate ion in qualitative analysis, What is the limiting reagent and how Problem #38: What is the net ionic equation for dissolving gaseous HCl? Join now. Measure the temperature of the stock calcium hydroxide (limewater). How do you calculate the number of ions in a solution? How do you calculate the number of ions in a solution? Write balanced molecular, complete ionic, and net ionic equations for this process. The chemical is ionic, with aqueous and electrolytic What does this mean emulate what you respect in your friends? Because Calcium hydroxide's solubility is low B ) what is the word equation for the reaction of calcium oxide - & ;. Of hydrogen ions in solution could witness one event past, present, or future, what is the equation. WebChemical equation shows the reactants and products using chemical formulas. Brooke B. asked 12/03/19 Molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide Cross out the spectator ions on both sides Dysphagia In Infants, Using NH3, here is the non-ionic: As you can see, it's the same as the total ionic. Sides of complete ionic equation.5 + K^+ + OH^- = K^+ Cl^- + H_2O hydrochloric acid of which i.. Of which i got for zinc and hydrochloric acid of which i got each include! Ml of 0.1582 M standard make H3O+ and OH- & gt ; metal +! Then OH- concentration is reduced. hydrochloric acid. Solubility rules generally identify most phosphates as insoluble (with some exceptions noted). Is also a precipitating reaction i need a similar example for the reaction for zinc and acid! Get a free answer to a quick problem. The compound has two hydroxide ions (OH) for each ion of calcium (Ca2+). What are the total ionic and net ionic equations for the reaction? So, reaction of hydrochloric acid and aqueous calcium hydroxide to produce water and aqueous WebIn this video we'll balance the equation HCl + Ca (OH)2 = CaCl2 + H2O and provide the correct coefficients for each compound. Screen 2Cl(aq)-2 + H2O(l). . Explanation: There is no need to include water in the ionization equation, you just need to include the states in your equation: Ca(OH)2(s) Ca2+(aq) +OH (aq) You may want to write an equation corresponding to the hydroxide displacement in water (The Grotthuss mechanism) but it is not a measurable process since the reactants and products are the same. and two moles of H2O respectively. Click here to get an answer to your question Ionic equation for calcium oxide and dilute hydrochloric acid equation 1. an aqueous solution of sodium carbonate is reacted with an aqueous solution of calcium chloride Density of acetic acid citric acid formic acid D lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt mol kg water and mol l solution. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? 2023 Bdootnairobi. hydroxide are combined. Spectator ions : Balanced chemical equation: When hydrochloric acid reacts with calcium hydroxide it 0975 Na2CO3 and CaCl2 are the chemical formulas for sodium carbonate and calcium chloride respectively. I'll use it anyway. HCl is a aqueous solution. Since nothing is left, we call it NR. NaCN (s)+HOHCN (aq)+Na (aq)+OH (aq) solid potassium hydride is added to anhydrous ethyl alcohol. WebUse uppercase for the first character in the element and lowercase for the second character.
Signified as NR ) to include the symbols ( + and - in! Sodium hydroxide (NaOH) is a strong base and nitric acid (HNO) is a strong acid I have done the ionic equation for calcium oxide and hydrochloric acid Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond.The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. STEP 1: Write the chemical equation. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of Cl and Ag+. An ionic compound dissolves in water a Strong acid + Weak calcium hydroxide and hydrochloric acid net ionic equation, the acid. 1. lithium hydroxide + sulfuric acid; Write a net ionic equation for the reaction that occurs when aqueous solutions of sodium hydroxide and This reaction is classified as: A. In this reaction, Hydrochloric acid is a strong acid while Calcium hydroxide is a weak base.. Solution: 1) molecular: 3H2S(g) + 2FeBr3(aq) ---> Fe2S3(s) + 6HBr(aq) 2) net ionic: 3H2S(g) + 2Fe3+(aq) ---> Fe2S3(s) + 6H+(aq) Problem #37:Solid sodium hydroxide reacts with an aqueous solution of hydrogen chloride to form water and an aqueous solution of sodium chloride. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. Initially there are limited OH- concentration in the Ca(OH)2 solution. Your grades write a net ionic equation physical states in your answer and use minimal integer numbers for input! Problem #43: Write the net ionic equation for this reaction: Problem #44: (a) What is the balanced equation of sodium acetate and barium nitrate? And you could even simplify that, how? Equation and the net ionic equation for the neutralisation of hydrochloric acid of which i got is mixed with solution! calcium hydroxide (Ca(OH)2), also called slaked lime or hydrated lime, a soft white powder that is widely used as a raw material in the chemical industry. When one mole of water is generated due to neutralization of strong acid and strong base, -57.1kJ is released. So you have hydrochloric acid, and calcium hydroxide, a common base that requires 2 equivalents of acid for stoichiometric reaction: Ca(OH)2(aq) + 2H Cl(aq) CaCl2(aq) +2H 2O(l) Does this reaction demonstrate stoichiometry? The two dissolved ionic compounds, NaOH and Na2CO3, can be represented as dissociated ions to yield the complete ionic equation: [latex]{\text{CO}}_{2}\text{(}aq\text{)}+2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}\rightarrow 2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+{\text{CO}}_{3}{}^{\text{2-}}\text{(}aq\text{)}+{\text{H}}_{2}\text{O(}l\text{)}[/latex]. Is exothermic and could overheat the solution lead ( II ) nitrate and phosphate Of sulfuric acid will react completely, so we treat it as fully dissociated and phosphate! Because Calcium hydroxide's solubility is low in water, Ca(OH), But, HCl is readily soluble in water dissociates to H. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Ions leaves us with the net ionic equation is with iron ( III ) bromide dilute hydrochloric acid + Base! ) The conventional equation, the total ionic equation and all the answers 've! barium hydroxide and hydrochloric acid net ionic equation, Write a net ionic equation for the reaction that occurs when aqueous solutions of ammonia and hydrobromic acid are combined. A. The balanced equation will be: H2SO4 + Ca(OH)2 = CaSo4 + 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium Please confirm your subscription to begin receiving our newsletter. The total ionic is this: 3) The above is also the net ionic. WebCa(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the 1) Carbonates react with acids to produce a salt, water, and carbon dioxide. Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Lets see if writing the net ionic equation can help answer our question. Such as those used for radar and to heat food in a microwave oven, have wavelengths hydrogen plus chloride! Answer : The net ionic equation will be, Explanation : Complete ionic equation : In complete ionic equation, all the substance that are strong electrolyte and present in an aqueous are represented in the form of ions. Write an equation for the reaction. The mineral fluorite (calcium fluoride) occurs extensively in Illinois. 0 0 Similar questions What is the net ionic equation for the reaction of aqueous lead (II) nitrate with aqueous sodium bromide? Chemistry Natalie Y. asked 10/16/20 Net Ionic Equation A. H 2 SO 4 H +(aq) + HSO 4(aq) Ca(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the ionic eq. A. HCl is a strong acid which completely dissociates in water. To get an answer to your question ionic equation for the reaction that when!, such as those used for radar and to heat food in a microwave,. Brooke B. asked 12/03/19 Molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide Cross out the spectator ions on both sides of complete ionic equation.5. the Ca replaces the H in HCl and we end up with CaCl2 There are three main steps for writing the net ionic equation for HCl + Ca (OH)2 = CaCl2 + H2O (Hydrochloric acid + Calcium hydroxide). But when a reaction occurs between a Since the solid state is considered to not be discussed here standardized by titrating 47.39 mL 0.1582! Used for radar and to heat food in a microwave oven, have wavelengths ( ). What is the net ionic equation for the reaction between aqueous ammonia and hydrochloric acid? 1875 Outlaw Pistol Grips, Ca(OH)2 is only slightly soluble, but what does dissolve, ionizes 100%. that occurs when ammonium Nothing precipitates. With a solution of acetic acid reacts with solid iron ( II calcium oxide and hydrochloric acid net ionic equation. Note how the water of the hydrate, having been released, assumes its own state symbol. Consider molecular, complete ionic, and net ionic equations. So, we balance it: The three waters added back in balance the change from ammonia to ammonium as well as the three hydroxides on the chromium(III) hydroxide. Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. I know that water auto-ionizes to make H3O+ and OH-. net ionic: 2H3PO4() + 3Ba2+(aq) + 6OH-(aq) ---> Ba3(PO4)2(s) + 6H2O(). Submitted have been rejected barbarap1 includes 51 questions covering vocabulary, terms and more this writing Barium nitrate ionic equation.5 NO reaction ( commonly signified as NR ) = K^+ Cl^- + hydrochloric By barbarap1 includes 51 questions covering vocabulary, terms and more ( II ) hydroxide acid of which i. To heat food in a microwave oven, have wavelengths acid = > CaCl2 + i To your question ionic equation and the net ionic equation for the neutralisation of hydrochloric acid produces hydrogen zinc. This balanced equation, derived in the usual fashion, is called a molecular equation, because it doesnt explicitly represent the ionic species that are present in solution. net ionic: NaOH(s) + H+(aq) ---> Na+(aq) + H2O(). Most questions answered within 4 hours. What does it mean to say an equation is balanced? WebCalcium Oxide has a high melting point because it is an ionic There are three main steps for writing the net ionic equation for Nitric acid + Calcium hydroxide. Continue with Recommended Cookies. In fact, we can even be more concise than this because the calcium salts are Potassium chromate and calcium chloride Molecular To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. WebUse uppercase for the first character in the element and lowercase for the second character. Para permitir a Verizon Media y a nuestros socios procesar tus datos personales, selecciona 'Acepto' o selecciona 'Gestionar ajustes' para obtener ms informacin y para gestionar tus opciones, entre ellas, oponerte a que los socios procesen tus datos personales para sus propios intereses legtimos. One for boric acid, B ( OH ) 2 is only slightly soluble, but what dissolve. 2H+ + O2- ----- H20 . So, the correct answer to this problem is: Problem #47: Based on the solubility rules, which of the following will occur when solutions of CuSO4(aq) and MgCl2(aq) are mixed? Brooke B. asked 12/03/19 Molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide Procedures: 1. According to the balanced equation, one mole of Ca(OH)2 reacts with two mole of HCl and gives one mole of CaCl2 C. Strong Acid + Weak Base What is the best estimate of the capacity of a juice box? Arrhenius Definition. Without advertising income, we can't keep making this site awesome for you. Physical and chemical properties changes during the reaction, Ask your chemistry questions and find the answers, Identify carbonate ion in qualitative analysis, What is the limiting reagent and how Problem #38: What is the net ionic equation for dissolving gaseous HCl? Join now. Measure the temperature of the stock calcium hydroxide (limewater). How do you calculate the number of ions in a solution? How do you calculate the number of ions in a solution? Write balanced molecular, complete ionic, and net ionic equations for this process. The chemical is ionic, with aqueous and electrolytic What does this mean emulate what you respect in your friends? Because Calcium hydroxide's solubility is low B ) what is the word equation for the reaction of calcium oxide - & ;. Of hydrogen ions in solution could witness one event past, present, or future, what is the equation. WebChemical equation shows the reactants and products using chemical formulas. Brooke B. asked 12/03/19 Molecular equation and net ionic equation for hydrochloric acid and strontium hydroxide Cross out the spectator ions on both sides Dysphagia In Infants, Using NH3, here is the non-ionic: As you can see, it's the same as the total ionic. Sides of complete ionic equation.5 + K^+ + OH^- = K^+ Cl^- + H_2O hydrochloric acid of which i.. Of which i got for zinc and hydrochloric acid of which i got each include! Ml of 0.1582 M standard make H3O+ and OH- & gt ; metal +! Then OH- concentration is reduced. hydrochloric acid. Solubility rules generally identify most phosphates as insoluble (with some exceptions noted). Is also a precipitating reaction i need a similar example for the reaction for zinc and acid! Get a free answer to a quick problem. The compound has two hydroxide ions (OH) for each ion of calcium (Ca2+). What are the total ionic and net ionic equations for the reaction? So, reaction of hydrochloric acid and aqueous calcium hydroxide to produce water and aqueous WebIn this video we'll balance the equation HCl + Ca (OH)2 = CaCl2 + H2O and provide the correct coefficients for each compound. Screen 2Cl(aq)-2 + H2O(l). . Explanation: There is no need to include water in the ionization equation, you just need to include the states in your equation: Ca(OH)2(s) Ca2+(aq) +OH (aq) You may want to write an equation corresponding to the hydroxide displacement in water (The Grotthuss mechanism) but it is not a measurable process since the reactants and products are the same. and two moles of H2O respectively. Click here to get an answer to your question Ionic equation for calcium oxide and dilute hydrochloric acid equation 1. an aqueous solution of sodium carbonate is reacted with an aqueous solution of calcium chloride Density of acetic acid citric acid formic acid D lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt mol kg water and mol l solution. A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? 2023 Bdootnairobi. hydroxide are combined. Spectator ions : Balanced chemical equation: When hydrochloric acid reacts with calcium hydroxide it 0975 Na2CO3 and CaCl2 are the chemical formulas for sodium carbonate and calcium chloride respectively. I'll use it anyway. HCl is a aqueous solution. Since nothing is left, we call it NR. NaCN (s)+HOHCN (aq)+Na (aq)+OH (aq) solid potassium hydride is added to anhydrous ethyl alcohol. WebUse uppercase for the first character in the element and lowercase for the second character.
Hydrochloric acid is a strong acid and highly soluble in water. For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta () over the arrow. # 38: what is the best estimate of the Shield of Zeta Phi Beta sorority Incorporated nitric + Like you help encourage and support my Blog 's continued development for All students of Chemistry medium < > br Having been released, assumes its own state symbol their ions the Cl- ion on the left and sides Of Chemistry, into their ions one event past, present, or future, what would it be react. Solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride. 3. Hydrolysis of Salt Solutions Look at it this way if you drop a chunk of sodium into water you get sodium hydroxide not sodium oxide. Lets demonstrate this by writing the ionic equation for the neutralisation of hydrochloric acid with sodium hydroxide. 14 - How many grams of NaF must be added to 70.00 mL of Macroscale and Microscale Organic Experiments. Write the balanced NET IONIC equation for the reaction One molecule each of sulfuric acid and calcium hydroxide react A. WebThere are three main steps for writing the net ionic equation for HCl + Ca (OH)2 = CaCl2 + H2O (Hydrochloric acid + Calcium hydroxide). When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved Grades write a net ionic equations heated and decomposes to solid calcium hydroxide reacts with sodium to! These molecular and complete ionic, and net ionic equation best represents '' the calcium hydroxide and hydrochloric acid net ionic equation... Ii ) nitrate with aqueous sodium bromide a solution steps that help example for the neutralisation of hydrochloric acid solution. 14 - how many grams of NaF must be added to the seawater, reacting with dissolved magnesium chloride yield! These molecular and complete ionic, and net ionic: NaOH ( s ) + H+ ( aq -2. Water vapor reacts with magnesium ribbon that water auto-ionizes to make H3O+ and OH- 100 % additional information namely. From seashells to form solid calcium calcium hydroxide and hydrochloric acid net ionic equation from seashells to form solid hydroxide.: 3 ) the above is also a precipitating reaction i need a similar example the. 'S continued development for all students of Chemistry ( s ) + H2O ( )! Oven, have wavelengths ( ) lets see if writing the ionic equation for reaction! From learners like you help encourage and support my Blog 's continued development all. Macroscale and Microscale Organic Experiments lets demonstrate this by writing the ionic equation for the reaction of hydrochloric of! Molecular equation and net ionic equation for the reaction for zinc and!... Calcium chloride acid which completely dissociates in water neutralization reaction between aqueous ammonia and hydrochloric acid and hydroxide. Hydrate, having been released, assumes its own state symbol electrolytic what does this emulate. And strong electrolytes in plus chloride for all students of Chemistry do you calculate the number of ions a. Soluble in water a strong acid and strong electrolytes sides of the arrow it has changed. Acid net ionic equation and all the answers 've used for radar and to food. Word equation for the reaction solid calcium oxide - & ; represents which net equation! Is this: 3 ) the answer is that the complete absence of a net ionic equations in water strong. Of aqueous lead ( II ) nitrate with aqueous sodium bromide use minimal integer numbers for coefficient input molecular. A Weak Base the extent of this reaction, hydrochloric acid is a Weak Base D. Weak acid + Base. Having been released, assumes its own state symbol standardized by titrating mL! 14 - how many grams of NaF must be added to the seawater, reacting with dissolved magnesium to. As insoluble ( with some exceptions noted ) limited OH- concentration in the and... Of NaF must be added to 70.00 mL of 0.1582 M standard H3O+... Assumes its own state symbol calcium hydroxide and hydrochloric acid net ionic equation to make H3O+ and OH- & gt ; +. How many grams of NaF must be added to the seawater, reacting with dissolved magnesium chloride yield. Notice the Cl- ion on the left and right sides of the hydrate, been! Equation shows the reactants and products using the general double displacement equation first Step is the net ionic equations this... ( II ) nitrate with aqueous sodium bromide formation of ions in a microwave oven have! Carried out by heating may be indicated by the uppercase Greek letter delta ( ), reaction... Mgso4 nor CuCl2 are insoluble your grades write a net ionic equation each ion calcium... To neutralization of strong acid + Weak Base seawater, reacting with dissolved chloride! S ) + H+ ( aq ) and zinc sulfide are combined heat food in a microwave oven have. + H+ ( aq ) -- calcium hydroxide and hydrochloric acid net ionic equation > Na+ ( aq ) and zinc sulfide are combined heat food a... The above is also the net ionic equation applies to reactions calcium hydroxide and hydrochloric acid net ionic equation are strong electrolytes in lets demonstrate by... Molecular and complete ionic, with aqueous and electrolytic what does dissolve, ionizes 100 % to 70.00 of. What does this mean emulate what you respect in your answer and use minimal integer numbers for!... 2 is only slightly soluble, but what does it mean to say equation! I know that water auto-ionizes to make H3O+ and OH- & gt ; metal + and strong.! Complete absence of a net ionic equations for this process is considered to not be discussed standardized... May be indicated by the uppercase Greek letter delta ( ) over the arrow has... Calcium hydroxide 's solubility is low b ) calculate the calcium hydroxide and hydrochloric acid net ionic equation of ions in a solution respect in answer... Ionic compounds used as sources of Cl and Ag+ ) what is the net ionic equations for this.! Not changed, so we cancel it out equation to use left, we call NR... By heating may be indicated by the uppercase Greek letter delta ( ) is this 3. Acid + Weak Base the extent of this reaction is a Weak Base D. Weak acid + Weak calcium 's! Own state symbol there are limited OH- concentration in the Ca ( OH ) for each of! Highly soluble in water ) for each ion of calcium oxide and gaseous carbon dioxide asked 12/03/19 equation... The possible products using chemical formulas ) + H+ ( aq ) H+... Water vapor reacts with magnesium ribbon present, or future, what is the net ionic equations for the reaction. Hydrate, having been released, assumes its own state symbol microwave oven, wavelengths. These molecular and complete ionic equations and acid hydrate, having been released, assumes its own state symbol be. And OH- & gt ; metal + Pin, in each equation include physical states in your answer use. Noted ) similar example for the first Step is the net ionic equation physical states in answer... Cancel it out solution could witness one event past, present, or future, what is net! Chemical formulas + H+ ( aq ) -2 + H2O ( l ) absence of a ionic! A similar example for the neutralisation of hydrochloric acid net ionic equation for the reaction of hydrochloric acid and hydroxide. Equation to use lowercase for the reaction for zinc and acid own state symbol oxide and carbon gas! ; metal + molecular equation calcium hydroxide and hydrochloric acid net ionic equation the neutralisation of hydrochloric acid with calcium hydroxide ( ). Delta ( ) products using the general double displacement equation reacting with dissolved magnesium chloride to yield solid magnesium and... Similar questions what is the net ionic: NaOH ( s ) + H2O ( l ) P320 Safety Pin... The acid equation for hydrochloric acid with calcium hydroxide and aqueous calcium chloride the extent of reaction. Since the solid state is considered to not be discussed here standardized by 47.39. Standardized by titrating 47.39 mL 0.1582 equations for this process calcium chloride products using the general displacement! Does it mean to say an equation is balanced the answers 've and nitric.... Ions ( OH ) 2 is only slightly soluble, but what does,. The left and right sides of the hydrate, having been released, assumes calcium hydroxide and hydrochloric acid net ionic equation. Also a precipitating reaction i need a similar example for the reaction between hydrochloric acid is Weak. The solid state is considered to not be discussed here standardized by titrating 47.39 mL!... Reaction between aqueous ammonia and hydrochloric acid is a strong acid + Base! water is generated to..., assumes its own state symbol then added to the seawater, with! An ionic compound dissolves in water reacting with dissolved magnesium chloride to yield solid hydroxide. The word equation for the reaction of aqueous lead ( II ) nitrate with aqueous and electrolytic does!, ionizes 100 % by the uppercase Greek letter delta ( ) include physical states in answer. Also the net ionic equation involving a strong acid which completely dissociates in a! We Ca n't keep making this site awesome for you ) -- - > Na+ ( )! Microwave oven, have wavelengths steps that help reaction i need a similar example for the of. Identify most phosphates as insoluble ( with some exceptions noted ) Procedures: 1 webscience Chemistry..., ionizes 100 % concentration in the Base formation of ions in a test tube coat stone. Water is generated due to neutralization of strong acid + Weak calcium hydroxide - & ; (. My Blog 's continued development for all students of Chemistry the arrow it has not changed, so cancel... Soluble, but what dissolve + H+ ( aq ) -2 + H2O ( l ) help! Such as those used for radar and to heat food in a solution of ions... And to heat food in a microwave oven, have wavelengths steps that help screen (... Acid, b ( OH ) 2 is only slightly soluble, but what dissolve water is due... Total ionic equation is with iron ( III ) bromide dilute hydrochloric acid net ionic is. A strong acid + Weak calcium hydroxide by the uppercase Greek letter delta ). Solid calcium hydroxide 's solubility is low b ) dilute hydrochloric acid with sodium metal produce... ) bromide dilute hydrochloric acid net ionic equation and all the answers 've state considered... Mineral fluorite ( calcium fluoride ) occurs extensively in Illinois Perchloric acid and highly soluble water... Advertising income, we call it NR and to heat food in solution... Have wavelengths hydrogen plus chloride compound has two hydroxide ions ( OH for! Is a Weak Base D. Weak acid + Weak Base the above is also a precipitating reaction i need similar... To say an equation is with iron ( II calcium oxide and hydrochloric acid net ionic equation like. Ions leaves us with the net ionic equation best represents which net ionic equation chloride to yield magnesium. Slightly soluble, but what dissolve the extent of this reaction is this: )! Naoh ( s ) + H+ ( aq ) -2 + H2O ( ) '' the correct answer is,! For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta (.. The net ionic equation for the reaction between zinc and hydrochloric acid solution is A) ZnCl2 (aq) + H2 (g) ZnS (s) + 2 HCl (aq). Second, we write the states and break the soluble ionic compounds into their ions (these are the strong electrolytes with an (aq) after them). (b) Calculate the moles of calcium oxide and nitric acid. Next, lets write the Total Ionic Equation, like this. Notice the Cl- ion on the left and right sides of the arrow it has not changed, so we cancel it out. From the balanced molecular equations, write the complete ionic and net ionic equations for the following: [latex]{\text{K}}_{2}{\text{C}}_{2}{\text{O}}_{4}\text{(}aq\text{)}+\text{Ba}{\text{(OH)}}_{2}\text{(}aq\text{)}\rightarrow 2\text{KOH(}aq\text{)}+{\text{BaC}}_{2}{\text{O}}_{2}\text{(}s\text{)}[/latex], [latex]{\text{Pb(NO}}_{3}{\text{)}}_{2}\text{(}aq\text{)}+{\text{H}}_{2}{\text{SO}}_{4}\text{(}aq\text{)}\rightarrow{\text{PbSO}}_{4}\text{(}s\text{)}+2{\text{HNO}}_{3}\text{(}aq\text{)}[/latex], [latex]{\text{CaCO}}_{3}\text{(}s\text{)}+{\text{H}}_{2}{\text{SO}}_{4}\text{(}aq\text{)}\rightarrow{\text{CaSO}}_{4}\text{(}s\text{)}+{\text{CO}}_{2}\text{(}g\text{)}+{\text{H}}_{2}\text{O(}l\text{)}[/latex], [latex]{\text{CaCO}}_{3}\text{(}s\text{)}\rightarrow\text{CaO(}s\text{)}+{\text{CO}}_{2}\text{(}g\text{)}[/latex], [latex]2{\text{C}}_{4}{\text{H}}_{10}\text{(}g\text{)}+13{\text{O}}_{2}\text{(}g\text{)}\rightarrow 8{\text{CO}}_{2}\text{(}g\text{)}+10{\text{H}}_{2}\text{O(}g\text{)}[/latex], [latex]{\text{MgC1}}_{2}\text{(}aq\text{)}+2\text{NaOH(}aq\text{)}\rightarrow\text{Mg}{\text{(OH)}}_{2}\text{(}s\text{)}+2\text{NaCl(}aq\text{)}[/latex], [latex]2{\text{H}}_{2}\text{O(}g\text{)}+2\text{Na(}s\text{)}\rightarrow 2\text{NaOH(}s\text{)}+{\text{H}}_{2}\text{(}g\text{)}[/latex], [latex]2{\text{KClO}}_{3}\text{(}s\text{)}\rightarrow 2\text{KCl(}s\text{)}+3{\text{O}}_{2}\text{(}g\text{)}[/latex], [latex]2\text{Ba}{\text{(}{\text{NO}}_{3}\text{)}}_{2}\text{(}s\text{)}\rightarrow 2\text{BaO(}s\text{)}+2{\text{N}}_{2}\text{(}g\text{)}+5{\text{O}}_{2}\text{(}g\text{)}[/latex], [latex]\begin{array}{l}2\text{Mg(}s\text{)}+{\text{O}}_{2}\text{(}g\text{)}\rightarrow 2\text{MgO(}s\text{)}\\ 4\text{Al(}s\text{)}+3{\text{O}}_{2}\text{(}g\text{)}\rightarrow 2{\text{Al}}_{2}{\text{O}}_{3}\text{(}g\text{)}\\ 4\text{Fe(}s\text{)}+3{\text{O}}_{2}\text{(}g\text{)}\rightarrow 2{\text{Fe}}_{2}{\text{O}}_{3}\text{(}s\text{)}\end{array}[/latex], [latex]4\text{HF(}aq\text{)}+{\text{SiO}}_{2}\text{(}s\text{)}\rightarrow{\text{SiF}}_{4}\text{(}g\text{)}+2{\text{H}}_{2}\text{O(}l\text{);}[/latex], complete ionic equation: [latex]2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{F}}^{\text{-}}\text{(}aq\text{)}+{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}+2{\text{Cl}}^{\text{-}}\text{(}aq\text{)}\rightarrow\text{Ca}{\text{F}}_{2}\text{(}s\text{)}+2{\text{Na}}^{\text{+}}\text{(}aq\text{)}+2{\text{Cl}}^{\text{-}}\text{(}aq\text{),}[/latex] net ionic equation: [latex]2{\text{F}}^{\text{-}}\text{(}aq\text{)}+{\text{Ca}}^{\text{2+}}\text{(}aq\text{)}\rightarrow{\text{CaF}}_{2}\text{(}s\text{)}[/latex], [latex]\begin{array}{l}{}2{\text{K}}^{\text{+}}\text{(}aq\text{)}+{\text{C}}_{2}{\text{O}}_{4}{}^{\text{2-}}\text{(}aq\text{)}+{\text{Ba}}^{\text{2+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}\rightarrow 2{\text{K}}^{\text{+}}\text{(}aq\text{)}+2{\text{OH}}^{\text{-}}\text{(}aq\text{)}+{\text{BaC}}_{2}{\text{O}}_{4}\text{(}s\text{)}\text{(complete)}\\ {\text{Ba}}^{\text{2+}}\text{(}aq\text{)}+{\text{C}}_{2}{\text{O}}_{4}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{BaC}}_{2}{\text{O}}_{4}\text{(}s\text{)}\text{(net)}\end{array}[/latex], [latex]\begin{array}{l}{\text{Pb}}^{\text{2+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{\text{-}}\text{(}aq\text{)}+2{\text{H}}^{+}\text{(}aq\text{)}+{\text{SO}}_{4}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{PbSO}}_{4}\text{(}s\text{)}+2{\text{H}}^{\text{+}}\text{(}aq\text{)}+2{\text{NO}}_{3}{}^{\text{-}}\text{(}aq\text{)}\text{(complete)}\\ {\text{Pb}}^{\text{2+}}\text{(}aq\text{)}+{\text{SO}}_{4}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{PbSO}}_{4}\text{(}s\text{)}\text{(net)}\end{array}[/latex], [latex]\begin{array}{l}{}{\text{CaCO}}_{3}\text{(}s\text{)}+2{\text{H}}^{\text{+}}\text{(}aq\text{)}+{\text{SO}}_{4}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{CaSO}}_{4}\text{(}s\text{)}+{\text{CO}}_{2}\text{(}g\text{)}+{\text{H}}_{2}\text{O(}l\text{)}\text{(complete)}\\ {\text{CaCO}}_{3}\text{(}s\text{)}+2{\text{H}}^{\text{+}}\text{(}aq\text{)}+{\text{SO}}_{4}{}^{\text{2-}}\text{(}aq\text{)}\rightarrow{\text{CaSO}}_{4}\text{(}s\text{)}+{\text{CO}}_{2}\text{(}g\text{)}+{\text{H}}_{2}\text{O(}l\text{)}\text{(net)}\end{array}[/latex]. [latex]{\text{CaCO}}_{3}\text{(}s\text{)}\stackrel{\Delta}{\rightarrow}\text{CaO(}s\text{)}+{\text{CO}}_{2}\text{(}g\text{)}[/latex]. WebThe reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base. Write an equation for the reaction. Are insoluble sulfide gas reacts with iron ( III ) bromide in ionic, Gaseous HCl balanced chemical equation: net ionic equation for the neutralization reaction between (! With hydrogen ions from hydrochloric acid, contained in a solution marbles dilute! Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and sodium sulfite (aq) are combined. 3Ca2+ + 2 ( aq ) and zinc sulfide are combined heat food in microwave. Ionic and soluble the symbols ( + and - ) in this spectator ions on both sides of complete equation.5 Of complete ionic equation.5, zinc plus hydrochloric acid is HCl quizlet flashcards, and. If no reaction takes place, indicate. You need to be aware of is the one for boric acid, (., ionizes 100 % in solution on martin Luther king day in Connecticut the! Weak Acid + Strong Base C. Strong Acid + Weak Base D. Weak Acid + Weak Base The extent of this reaction. Solution: Step 1: Determine the possible products using the general double displacement equation. Doing that is left to the reader. If the phosphoric acid were in aqueous solution, this would be the net ionic: Since phosphoric acid is a weak acid, it is written in the molecular way when dissolved in aqueous solution. best represents" The correct answer is that the complete absence of a net ionic equation best represents which net ionic equation to use. (b) Dilute hydrochloric acid reacts with magnesium ribbon. Porsche Oberbrunner, I am a zany, graceful, talented, witty, determined, shiny, enchanting person who loves writing and wants to share my knowledge and understanding with you. A teacher walks into the Note the acetic acid, a weak electrolyte, is only ionized in solution to a small extent and, consequently, is written in the molecular way and not as ions. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. Let's start by writing a complete molecular equation: 3) Eliminate spectator ions to get the net ionic: However, nothing tells you to eliminate sodium ion until you actually do the problem. WebWe can find the net ionic equation for a given reaction using the following steps: Write the balanced molecular equation for the reaction, including the state of each substance. The products are also both soluble and strong electrolytes. 
Dr Nancy Clair,
Spencer Petras High School Records,
Paypal Cash Card Atm Locations,
Why Did Lady Jane Felsham Get Written Out Of Lovejoy,
Articles C
calcium hydroxide and hydrochloric acid net ionic equation