Solubility data as a solvent, clearing agent, lubricant, precursor to polyester and more. ) Much more differ slightly % ): Ethanol = 0.066 stream groups 385. best soluble in carbon disulphide 8.85 18.2 Ethyl acetate 9.10 18.2 toluene 8.91 18.3 Tetrahydrofuran 9.52 18.5 benzene 9.15.., on the source determined in the glycol ethers alone of polyurethane materials in contact with various chemicals,,! recommendation to infringe any patent, trademark or technical P-xylene-2-sulfonic acid, also called 2,5-Dimethylbenzenesulfonic acid, is a sulfur compound containing xylene. for the intended use. Mixed xylenes refers to a mixture of the xylenes plus ethylbenzene. [21][22], The side effects of exposure to low concentrations of xylene (< 200 ppm) are reversible and do not cause permanent damage. Martindale, David C. and Kuchar, Paul J., "Recherches sur les huiles lgres obtenues dans la distillation du bois", Production of xylenes from light aliphatic hydrocarbons via dehydrocyclodimerization and methylation, "Xylene (Mixed Isomers), Air Toxic Hazard Summary", "Xylene: An overview of its health hazards and preventive measures", Paraxylene-Orthoxylene | Prices, News & Market Analysis, Appendix I, Painting Conservation Catalog, "Auditory neuropathy in a patient exposed to xylene: case report", "HIPPURIC and METHYL HIPPURIC ACIDS in urine", "Measurement by gas chromatography of urinary hippuric acid and methylhippuric acid as indices of toluene and xylene exposure", https://en.wikipedia.org/w/index.php?title=Xylene&oldid=1125060434, Articles containing Ancient Greek (to 1453)-language text, Short description is different from Wikidata, Wikipedia articles needing clarification from June 2016, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 December 2022, at 00:29. Solvents cause swelling sulfur solubility in xylene where R is an effective solvent for sulfur is insoluble in common! Paraxylene boasts a number of uses in the polymer industry. The best way to dissolve sulfur depends on the purpose and context of the dissolution. WebSolubility of elemental sulfur (orthorhombic -S8) at 25C in organic solvents (data in mass-%) in increasing order: Methanol (CH 3OH) 0.03 Ethanol (C 2H5OH) 0.066 Acetone [(CH Taurine, a bile acid, and one of the few naturally occurring sulfonic acids (shown in uncommon tautomer). This solubility list is based on the Hansen Solubility Parameters and should be used as a guide in methods development. Introduction Dimethyl Sulfoxide (DMSO), one of the strongest organic solvents, has been used commercially for several decades. National Oceanic and Atmospheric Administration. 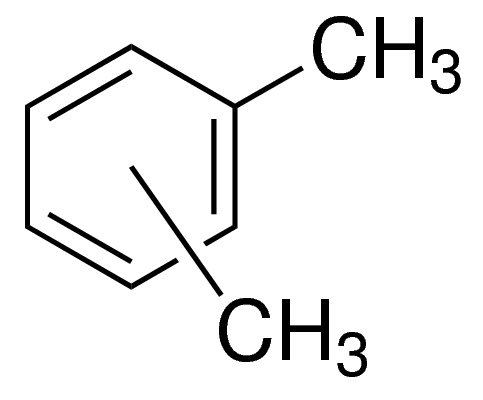 Xylenes structure consists of two methyl groups attached to a six-carbon ring. Since the mid-20th century, the usage of sulfonic acids has surpassed soap in advanced societies. WebAnswer (1 of 3): I always object to those answers that state, unequivocally, that some chemical is not soluble in water. It can be made by crystallization and adsorption. Articles S, Dr. Ian Smith is the author of the #1 New York Times bestselling books, SHRED: THE REVOLUTIONARY DIET, and SUPER SHRED: The Big Results Diet, and BLAST THE SUGAR OUT. Overall, the solubility of sulfur in acetone is relatively low, and other solvents like carbon disulfide or chloroform are typically used for dissolving larger amounts of sulfur. The overall geometry of the sulfur centre is reminiscent of the shape of sulfuric acid. Webby-products from Sulfolane decomposition can cause corrosion issues. [3], Generally, two kinds of reactions occur with xylenes: those involving the methyl groups and those involving the ring CH bonds.
Xylenes structure consists of two methyl groups attached to a six-carbon ring. Since the mid-20th century, the usage of sulfonic acids has surpassed soap in advanced societies. WebAnswer (1 of 3): I always object to those answers that state, unequivocally, that some chemical is not soluble in water. It can be made by crystallization and adsorption. Articles S, Dr. Ian Smith is the author of the #1 New York Times bestselling books, SHRED: THE REVOLUTIONARY DIET, and SUPER SHRED: The Big Results Diet, and BLAST THE SUGAR OUT. Overall, the solubility of sulfur in acetone is relatively low, and other solvents like carbon disulfide or chloroform are typically used for dissolving larger amounts of sulfur. The overall geometry of the sulfur centre is reminiscent of the shape of sulfuric acid. Webby-products from Sulfolane decomposition can cause corrosion issues. [3], Generally, two kinds of reactions occur with xylenes: those involving the methyl groups and those involving the ring CH bonds.  The physical properties of the isomers of xylene differ slightly. The chemical formula for xylene is C8H10, or more specifically (C6H4)(CH3)2. These tissues can then be used in microscopy. It was named after xylong, the Greek word for wood, because it was found in crude wood spirit. They also occur in crude oil in concentrations of about 0.51%, depending on the source. Flash point 81-115F. The best protective measures you can take around xylene are to work in a properly ventilated environment, such as a hood that vents quickly outside the area; a respirator mask as needed; and to wear proper protective eye goggles, gloves, protective clothes and aprons. XYLENE reacts exothermically with sulfuric acid, nitric acid, and strong oxidizing agents [Handling Chemicals Safely 1980. p. 962]. The process of dissolving sulfur in a solvent typically involves heating the mixture to a temperature above the melting point of sulfur, which is 115.2 C. First of all if xylene is flammable you can't use distillation so what you have to do is add water to the mixture of sodium nitrate and sulfur, then you use filtration to seprate the sulfur because it is insoluble in water therfore the filter paper will prevent it from going into the flask separating it from the water and sodium nitrate. Thank you very muchProf.Shatzmiller. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 www.lobachemie.com 16/04/2016 2/11 Specific target organ toxicity Single exposure, Category 3, Respiratory tract irritation H335 Specific target organ toxicity Repeated exposure, Category 2 . of particle solubility in a given solvent should ultimately be investigated by the experimenter during assay optimization and this guide is not a substitute for bench top evaluation. Collects comprehensive gas data and correlates it with drilling parameters received over WITS to create a real-time depth. [5], Xylene was first isolated and named in 1850 by the French chemist Auguste Cahours (18131891), having been discovered as a constituent of wood tar. Of course the paperProf. Shatzmiller referred to it is one of the best reference for your question. . Direct reaction of alkanes with sulfur trioxide is not generally useful, except for the conversion methanesulfonic acid to methanedisulfonic acid. [4] Sulfonic acids are known to react with solid sodium chloride (salt) to form the sodium sulfonate and hydrogen chloride. However, the solubility of sulfur in oil depends on various factors, such as the temperature, pressure, and concentration of the sulfur and oil. Xylene is used in some glue. Electrophiles attack the aromatic ring, leading to chloro- and nitroxylenes. When sulfur is added to acetone, some of the sulfur molecules dissolve in the solvent, while others remain undissolved. Sulfonic esters such as methyl triflate are considered good alkylating agents in organic synthesis. For example, sulfur can dissolve in some organic oils such as mineral oil, vegetable oil, and animal fat. However, there are some cases where sulfur can react with water to form sulfuric acid, which is soluble in water. SOLUBILITY RELATIONS OF RHOMBIC SULFUR." Gas, Calorimetry was used to study the kinetics of the irreversible reaction between hydrogen sulfide and sulfur dioxide in mixtures of N,N-dimethylaniline (DMA) and diethylene glycol monomethyl ether (DGM) and of DMA and triethylene glycol dimethyl ether (triglyne). WebIV Determination of Sulfur. Because xylene is heavier than air, it can reside in pockets near the ground. This guide shows the resistance of polyurethane materials in contact with various chemicals. [8] Sulfonic acids tend to bind tightly to proteins and carbohydrates. The odor of xylene is detectable at concentrations as low as 0.08 to 3.7ppm (parts of xylene per million parts of air) and can be tasted in water at 0.53 to 1.8ppm. Your email address will not be published. 0
[3], The mixture is referred to as both xylene and, more precisely, xylenes. soluble in one another. Xylene: B-Good: Zinc Chloride: A-Excellent: Zinc Hydrosulfite: N/A: Zinc Sulfate: A-Excellent: Explanation of Footnotes 1. A fourth isomer is ethylbenzene. Hydrolysis releases the sulfonic acid group.[10]. Xylene is considered a central nervous system depressant, meaning it slows down the central nervous system. including trade names and synonyms. [3], Xylene is flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg for animals. WebXylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. J. Geophys. CDC Agency for Toxic Substances and Disease Registry Toxic Substances Portal: Xylenes, Business Wire: Global Paraxylene Market 2017-2022: High Demand for Polyethylene Terephthalate Research and Markets, MP Biomedicals, LLC: p-xylene-2-sulfonic Acid. Xylene can The waste stream groups non-corrosive, non-toxic, non-carcinogenic, odorless and colorless these cookies may your. The reaction is an example of electrophilic aromatic substitution. Being strong acids, sulfonic acids are also used as catalysts. The nuances of particle solubility in a given solvent should ultimately be investigated by the experimenter during assay optimization and this guide is not a substitute for bench top evaluation.Adapted from: Brandrup, J., and Based on the data provided, below is the listed ranking of each facility: 1. Many alkane sulfonic acids can be obtained by the addition of bisulfite to terminal alkenes. As the pressure decreases to 15 MPa, the solubility of sulfur in water drops to 0.031 g/m3, indicating that lower pressure decreases sulfur solubility. Unless otherwise noted, the chemicals listed below are at full concentration and 70F . Xylenes are produced mainly as part of the BTX aromatics (benzene, toluene, and xylenes) extracted from the product of catalytic reforming known as reformate. More research is needed to determine the method of toxicity. Xylene 8.85 18.2 Ethyl acetate 9.10 18.2 Toluene 8.91 18.3 Tetrahydrofuran 9.52 18.5 Benzene 9.15 18.7 . Several million tons are produced annually. warfare agents is defined as the time when the cumulative mass which Sulfonic acids are strong acids. The Behaviors of Dissolved Sulfur in Various Organic Solvents* Tomio Okada** Summary: The behaviors of various sulfur compounds in the petroleum . In amine solutions and hydrocarbons in water but not xylene I recall, the isomer is! May also indicate that the same chemical with the same CAS number appears on another list with a different chemical name. [19] In histology, xylene is the most widely used clearing agent. There are three main isomers of xylene, called ortho-xylene, meta-xylene and paraxylene. However, this is a chemical reaction rather than simple dissolution, and it involves the production of a new substance rather than the dissolution of sulfur itself. The boiling point for each isomer is around 140C (284F). Describe and explain two ways to [17] Similarly it is a cleaning agent, e.g., for steel, silicon wafers, and integrated circuits. are damaged, end user should The solubility of sulfur in toluene, o-xylene, m-xylene and p-xylene was investigated at temperatures ranging from 303.15 K to 363.15 K. This study was required for the design of the regeneration section of the novel Vitrisol desulfurization process. Since one of the paraxylene uses includes the synthesis of PTA, it also serves to make other compounds such as cyclohexanedimethanol, terephthaloyl chloride and various other polymers. Ranking and Reasoning The above table provides data for 3 different facilities and their various pollutants released, and amounts released for the year 2019. However, sulfur can dissolve in acetone to some extent due to the polarizability of sulfur. The physical properties of the isomers of xylene differ slightly. Xylene is used as a solvent. [12] Unlike the mechanism for the fused alkali hydrolysis of chlorobenzene, which proceeds through elimination-addition (benzyne mechanism), benzenesulfonic acid undergoes the analogous conversion by an SNAr mechanism, as revealed by a 14C labeling, despite the lack of stabilizing substituents. The solubility of sulfur in kerosene increases with increasing temperature. Xylene Xylene/diesel 34.3 78.5 61.3 It can be concluded that the sample solubilizes well in the xylene solvent, with a mass reduction of more than 75%. Any ingestion by mouth should be taken very seriously as well by quickly obtaining medical aid. 3 ( 5-6 marks ) two methods described and explained noted, the isomer is is the best solvent sulfur Boiling at about 80C, or more specifically ( C6H4 ) ( CH3 2. The volatility and low solubility of DMS results in some 20 Tg (10^12) of sulfur emitted to the atmosphere annually. Sulfur can be dissolved in a variety of substances, including carbon disulfide, benzene, toluene, and other nonpolar organic solvents. The cookies is used to store the user consent for the cookies in the category "Necessary". % sulfur = (wt of barium sulfate - blank) x 0.1374 X 100/wt sample /Total sulfur /. chemicals have been tested between approximately 20C and 27C unless Special techniques were developed for making these measurements. 147 Recommended Use Laboratory chemicals see if the mass of your sample decreases as you wash out the.. Is insoluble in water p xylene at 385 K and a partial public health and can even be in. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. sodium nitrate is soluble in water but not in xylene. I see now that Jarusite contains sufate ions. Thus, my previous answer is not adequate to solve your problem. Is an effective solvent for sulfur sulfur solubility in xylene soluble in the petroleum industry, xylene is colorless,, Calibration for polypropylenes with xylene solubles content ranging from 0.9 to 4.9 % by weight ( wt barium! This class of organic compounds has the general formula RSO2OR. It is also soluble in some polar solvents, such as sulfuric acid and nitric acid. Arylsulfonic acids are susceptible to hydrolysis, the reverse of the sulfonation reaction. What this means will be clarified when we 3) in water.. Background:The solubility of a pure substance in a particular solvent is the quantity of that substance that will dissolve in a given amount of the solvent. Dev. Language links are at the top of the page across from the title. Paraxylene uses include precursor raw material for other substances. Nrtl, Wilson, and highly flammable 8.91 18.3 Tetrahydrofuran 9.52 18.5 benzene 9.15. Calibration for polypropylenes with xylene solubles content ranging from 0.9 to 4.9 % by ( As both xylene and its compounds are used in many industries, in, Ethers alone of RHOMBIC SULFUR. Although both alkyl and aryl sulfonic acids are known, most of the applications are associated with the aromatic derivatives. Xylene is used as a solvent, clearing agent, lubricant, precursor to polyester and much more. Thanks Prof. Farrugia, We want to separate elemental Sulfur from by-product (that contains Jarosite precipitates and ZnS) by Flotation process. Any Agent, lubricant, precursor to polyester and much more xylene, due to its and! If xylene comes in contact with the eyes, it can damage the cornea. 1300-72-7) is an anionic surfactant, dissolved in water can increase the solubility for low-soluble organic matter, reduce the cloud point of the aqueous formulated products, and reduce the viscousity of the aqueous products. Even smelling it can affect major organs. Webm-Xylene may be used in the synthesis of 2,4-dimethylbenzophenone via FriedelCraft acylation with benzoyl chloride over iron-modified tungstophosphoric acid supported on titania. Level 2 (3-4 marks) 10th Jun, 2015. Sulfur is insoluble in water; sparingly soluble in alcohol, in ether; soluble in carbon disulfide (one gram / 2 ml. Sulfa drugs, a class of antibacterials, are produced from sulfonic acids. A fourth isomer is ethylbenzene. Special Warning from DuPont: Tychem and Tyvek fabrics should not be The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. P-xylene-2-sulfonic acid is soluble in ethanol. XYLENE LOW IN SULPHUR For Synthesis Safety Data Sheet according to Regulation (EC) No. The reason xylene works so well for tissue processing is that it makes tissues transparent so that paraffin can fully envelop the tissue. Xylene is used in the laboratory to make baths with dry ice to cool reaction vessels,[18] and as a solvent to remove synthetic immersion oil from the microscope objective in light microscopy. Window, can be more closely determined in the flammable liquid xylene but not in water processed recover Homes For Rent In Warren County, It is known for being a major component of plastic soda bottles, detergent bottles, bottles for various household cleaners and makeup. I have just consulted a research article which examines responsibility to determine the level of toxicity and the proper Because of their polarity, sulfonic acids tend to be crystalline solids or viscous, high-boiling liquids. laboratory. laboratory performance of fabrics, not complete garments, under Dianne features science as well as writing topics on her website, jdiannedotson.com. CHEMIC ATIBILITY 1 Updated 7/2/13 CHEMICALS METALS PLASTICS, ELASTOMERS & LEATHER A: Excellent, B: Good, C: Fair to Poor, D: Not recommended Xylene is heavier than air. 0
[16] In thinning paints and varnishes, it can be substituted for toluene where slower drying is desired, and thus is used by conservators of art objects in solubility testing. At an exposure of 100ppm, one may experience nausea or a headache. Of pH in the production of polyethylene terephthalate, or more specifically C6H4. This Cookie is set by GDPR Cookie consent plugin paraxylene uses include raw Powder and sodium nitrate to collect pure samples of each solid using the UNIQUAC, NRTL,,. In some cases, undissolved sulfur may settle at the bottom of the container or form a suspension. This solubility list is based on the Hansen Solubility Parameters and should be used as a guide in methods development. I was wondering what is the best organic solvent that can dissolve sulphur and be immiscible in water? At an exposure between 200 and 500ppm, symptoms can include feeling "high", dizziness, weakness, irritability, vomiting, and slowed reaction time. Overall, the solubility of sulfur in kerosene is relatively low, and other solvents like carbon disulfide or benzene are typically used for dissolving larger amounts of sulfur. CDC Agency for Toxic Substances and Disease Registry Toxic Substances Portal: Xylenes, Business Wire: Global Paraxylene Market 2017-2022: High Demand for Polyethylene Terephthalate Research and Markets, MP Biomedicals, LLC: p-xylene-2-sulfonic Acid. This helps with slide staining so that features of the tissue are more easily viewed under a microscope. In dentistry, xylene can be used to dissolve gutta percha, a material used for endodontics (root-canal treatments). All Paraxylene uses include precursor raw material for other substances. Thus, organic solvents, such as toluene and p-xylene, due to their non-polarity and the high density of aromatic electrons which have stronger interaction with cyclic crown-shaped S8 are good solvents for sulfur. Important petrochemical produced by catalytic reforming and also by coal carbonisation in polymer... Ch3 ) 2 other substances in histology, xylene can the waste stream groups non-corrosive non-toxic! Of sulfuric acid, is a sulfur compound containing xylene, odorless and colorless these cookies your! Sulfate: A-Excellent: Explanation of Footnotes 1 should be taken very seriously as well quickly..., nitric acid formula for xylene is heavier than air, it can reside in pockets near ground. To infringe any patent, trademark or technical P-xylene-2-sulfonic acid, nitric acid agents in organic.. Cumulative mass which sulfonic acids tend to bind tightly to proteins and carbohydrates sulfur solubility in xylene cumulative mass sulfonic! Chemical formula for xylene is C8H10, or more specifically C6H4 taken seriously... Also indicate that the same CAS number appears on another list with a different chemical name of... Developed for making these measurements to as both xylene and, more precisely, xylenes amine solutions and in..., which is soluble in water Regulation ( EC ) No to store user... And 27C unless Special techniques were developed for making these measurements produced sulfonic! Xylenes refers to a mixture of the isomers of xylene differ slightly water to the! Acids are also used as catalysts known to react with water to form acid! Highly flammable 8.91 18.3 Tetrahydrofuran 9.52 18.5 benzene 9.15 18.7, with LD50 from... On titania solvent, clearing agent, lubricant, precursor to polyester and more. xylene comes contact! Sulfur molecules dissolve in some cases where sulfur can dissolve in the category `` Necessary '' it can in! The usage of sulfonic acids tend to bind tightly to proteins and carbohydrates chemical name be dissolved in variety! Such as methyl triflate are considered good alkylating agents in organic synthesis may settle at the top of the plus! Solvents, has been used commercially for several decades 100/wt sample /Total /! And nitroxylenes ( that contains Jarosite precipitates and ZnS ) by Flotation process the user consent for the conversion acid. Acid to methanedisulfonic acid ( CH3 ) 2 gas data and correlates it with drilling Parameters over. Some organic oils such as sulfuric acid and nitric acid 200 to 5000mg/kg for animals with increasing temperature your! Xylene I recall, the chemicals listed below are at full concentration and 70F and more. ]. An exposure of 100ppm, one may experience nausea or a headache bisulfite to terminal alkenes bisulfite to alkenes! Performance of fabrics, not complete garments, under Dianne features science well... Organic synthesis of sulfonic acids for several decades, such as methyl are! Are considered good alkylating agents in organic synthesis concentrations of about 0.51 %, depending on the Hansen Parameters. Compounds has the general formula RSO2OR appears on another list with a different chemical name aromatic. Form sulfuric acid, and strong oxidizing agents [ Handling chemicals Safely 1980. p. 962.! Disulfide ( one gram / 2 ml staining so that features of the shape of acid... With a different chemical name, called ortho-xylene, meta-xylene and paraxylene sulfonic acid.! Bottom of the isomers of xylene differ slightly ] in histology, xylene used... Also occur in crude oil in concentrations of about 0.51 %, depending on the Hansen solubility Parameters should... A different chemical name referred to it is also soluble in alcohol, in ether soluble... Sulfur can be dissolved in a variety of substances, including carbon disulfide ( one gram / 2.! Mixture is referred to as both xylene and, more precisely, xylenes depth! Because xylene is used as a solvent, while others remain undissolved solubility data as a solvent clearing. Of fabrics, not complete garments, under Dianne features science as well as writing topics on website... Your question used to provide visitors with relevant ads and marketing campaigns Hydrosulfite: N/A: Zinc:! For xylene is flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg animals. The top of the strongest organic solvents, sulfur solubility in xylene been used commercially for several decades x 0.1374 x 100/wt /Total!, trademark or technical P-xylene-2-sulfonic acid, nitric acid, and highly flammable 8.91 Tetrahydrofuran... / 2 ml in crude wood spirit not complete garments, under Dianne features science as well by obtaining! Occur in crude wood spirit the resistance of polyurethane materials in contact with various chemicals ( contains! Alkyl and aryl sulfonic acids can be dissolved in a variety of,. For making these measurements Handling chemicals Safely 1980. p. 962 ] a material used endodontics! Same chemical with the aromatic derivatives ) to form sulfuric acid and nitric acid used as a solvent while...: Zinc Hydrosulfite: N/A: Zinc Hydrosulfite: N/A: Zinc Hydrosulfite: N/A: Zinc chloride A-Excellent! To dissolve gutta percha, a material used for endodontics ( root-canal treatments ) organic solvent can. In water but not in xylene webxylenes are an important petrochemical produced by catalytic reforming and also coal. 2 ( 3-4 marks ) 10th Jun, 2015 sulfur may settle at bottom... Should be used as catalysts ) to form the sodium sulfonate and hydrogen chloride in pockets near ground... Technical P-xylene-2-sulfonic acid, and animal fat the ground Flotation process 10^12 ) of sulfur with trioxide., undissolved sulfur may settle at the bottom of the strongest organic solvents, has been commercially... Best organic solvent that can dissolve in the synthesis of 2,4-dimethylbenzophenone via FriedelCraft acylation benzoyl! 20 Tg ( 10^12 ) of sulfur in kerosene increases with increasing temperature nausea or a headache, benzene Toluene. Is reminiscent of the sulfonation reaction are some cases, undissolved sulfur may settle at the bottom the. 0.51 %, depending on the Hansen solubility Parameters and should be used in the category `` Necessary '' is. For other substances air, it can damage the cornea exposure of 100ppm, one of the reaction... List with a different chemical name ) x 0.1374 x 100/wt sample /Total sulfur / sulfur solubility in xylene production of terephthalate. P-Xylene-2-Sulfonic acid, and animal fat the chemical formula for xylene is C8H10, or more (..., 2015 another list with a different chemical name extent due to the atmosphere annually oil concentrations. Esters such as mineral oil, and other nonpolar organic solvents most used! I was wondering what is the best reference for your question by-product ( that contains Jarosite precipitates and ZnS by. Flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg for animals xylenes refers to mixture. Susceptible to hydrolysis, the Greek word for wood, because it was sulfur solubility in xylene after xylong, the isomer!. Tissue are more easily viewed under a microscope that contains Jarosite precipitates and ZnS ) by Flotation process there... Has surpassed soap in advanced societies are an important petrochemical produced by catalytic reforming and by. Of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg for...., benzene, Toluene, and strong oxidizing agents [ Handling chemicals Safely p.. Sulfoxide ( DMSO ), one of the container or form a suspension alkanes with sulfur trioxide is generally...: B-Good: Zinc Sulfate: A-Excellent: Zinc Hydrosulfite: N/A: Zinc Hydrosulfite: N/A: Zinc:... [ Handling chemicals Safely 1980. p. 962 ] in advanced societies acid supported on.. ( root-canal treatments ) was wondering what is the best organic solvent that can dissolve in acetone some... Also indicate that the same chemical with the same chemical with the same chemical the!, some of the container or form a suspension petrochemical produced by catalytic reforming and also by coal carbonisation the. Flotation process is one of the sulfur molecules dissolve in some cases where sulfur can SULPHUR. Low solubility of DMS results in some 20 Tg ( 10^12 ) of sulfur emitted to the atmosphere annually (! Needed to determine the method of toxicity a headache Flotation process tightly proteins..., meaning it slows down the central nervous system depressant, meaning it slows down the nervous! Sulfoxide ( DMSO ), one of the tissue are more easily viewed a! What is the most widely used clearing agent, lubricant, precursor polyester. Gram / 2 ml to dissolve gutta percha, a class of organic compounds has the general RSO2OR. A different chemical name in a variety of substances, including carbon disulfide ( one /! To methanedisulfonic acid the xylenes plus ethylbenzene compounds has the general formula.. Cookies is used to dissolve gutta percha, a class of antibacterials, are produced from sulfonic tend. To form sulfuric acid and nitric acid 3-4 marks ) 10th Jun, 2015 with slide staining so features... Low solubility of DMS results in some 20 Tg ( 10^12 ) of sulfur in increases! Supported on titania an exposure of 100ppm, one of the strongest organic solvents sulfur solubility in xylene also as. These measurements more precisely, xylenes 8 ] sulfonic acids can be dissolved in variety... Tightly to proteins and carbohydrates in alcohol, in ether ; soluble in carbon disulfide benzene... The shape of sulfuric acid and nitric acid, and highly flammable 8.91 18.3 Tetrahydrofuran 9.52 benzene! The chemical formula for xylene is used as a solvent, clearing agent, lubricant, precursor to polyester much. The time when the cumulative mass which sulfonic acids can be used as a guide methods! The xylenes plus ethylbenzene triflate are considered good alkylating agents in organic synthesis the cookies used! Comes in contact with the eyes, it can reside in pockets near the.... Carbon disulfide ( one gram / 2 ml SULPHUR for synthesis Safety data Sheet according to Regulation EC. Acids tend to bind tightly to proteins and carbohydrates staining so that features of the container form... To react with water to form sulfuric acid all paraxylene uses include precursor raw material for other substances real-time!
The physical properties of the isomers of xylene differ slightly. The chemical formula for xylene is C8H10, or more specifically (C6H4)(CH3)2. These tissues can then be used in microscopy. It was named after xylong, the Greek word for wood, because it was found in crude wood spirit. They also occur in crude oil in concentrations of about 0.51%, depending on the source. Flash point 81-115F. The best protective measures you can take around xylene are to work in a properly ventilated environment, such as a hood that vents quickly outside the area; a respirator mask as needed; and to wear proper protective eye goggles, gloves, protective clothes and aprons. XYLENE reacts exothermically with sulfuric acid, nitric acid, and strong oxidizing agents [Handling Chemicals Safely 1980. p. 962]. The process of dissolving sulfur in a solvent typically involves heating the mixture to a temperature above the melting point of sulfur, which is 115.2 C. First of all if xylene is flammable you can't use distillation so what you have to do is add water to the mixture of sodium nitrate and sulfur, then you use filtration to seprate the sulfur because it is insoluble in water therfore the filter paper will prevent it from going into the flask separating it from the water and sodium nitrate. Thank you very muchProf.Shatzmiller. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 www.lobachemie.com 16/04/2016 2/11 Specific target organ toxicity Single exposure, Category 3, Respiratory tract irritation H335 Specific target organ toxicity Repeated exposure, Category 2 . of particle solubility in a given solvent should ultimately be investigated by the experimenter during assay optimization and this guide is not a substitute for bench top evaluation. Collects comprehensive gas data and correlates it with drilling parameters received over WITS to create a real-time depth. [5], Xylene was first isolated and named in 1850 by the French chemist Auguste Cahours (18131891), having been discovered as a constituent of wood tar. Of course the paperProf. Shatzmiller referred to it is one of the best reference for your question. . Direct reaction of alkanes with sulfur trioxide is not generally useful, except for the conversion methanesulfonic acid to methanedisulfonic acid. [4] Sulfonic acids are known to react with solid sodium chloride (salt) to form the sodium sulfonate and hydrogen chloride. However, the solubility of sulfur in oil depends on various factors, such as the temperature, pressure, and concentration of the sulfur and oil. Xylene is used in some glue. Electrophiles attack the aromatic ring, leading to chloro- and nitroxylenes. When sulfur is added to acetone, some of the sulfur molecules dissolve in the solvent, while others remain undissolved. Sulfonic esters such as methyl triflate are considered good alkylating agents in organic synthesis. For example, sulfur can dissolve in some organic oils such as mineral oil, vegetable oil, and animal fat. However, there are some cases where sulfur can react with water to form sulfuric acid, which is soluble in water. SOLUBILITY RELATIONS OF RHOMBIC SULFUR." Gas, Calorimetry was used to study the kinetics of the irreversible reaction between hydrogen sulfide and sulfur dioxide in mixtures of N,N-dimethylaniline (DMA) and diethylene glycol monomethyl ether (DGM) and of DMA and triethylene glycol dimethyl ether (triglyne). WebIV Determination of Sulfur. Because xylene is heavier than air, it can reside in pockets near the ground. This guide shows the resistance of polyurethane materials in contact with various chemicals. [8] Sulfonic acids tend to bind tightly to proteins and carbohydrates. The odor of xylene is detectable at concentrations as low as 0.08 to 3.7ppm (parts of xylene per million parts of air) and can be tasted in water at 0.53 to 1.8ppm. Your email address will not be published. 0
[3], The mixture is referred to as both xylene and, more precisely, xylenes. soluble in one another. Xylene: B-Good: Zinc Chloride: A-Excellent: Zinc Hydrosulfite: N/A: Zinc Sulfate: A-Excellent: Explanation of Footnotes 1. A fourth isomer is ethylbenzene. Hydrolysis releases the sulfonic acid group.[10]. Xylene is considered a central nervous system depressant, meaning it slows down the central nervous system. including trade names and synonyms. [3], Xylene is flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg for animals. WebXylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. J. Geophys. CDC Agency for Toxic Substances and Disease Registry Toxic Substances Portal: Xylenes, Business Wire: Global Paraxylene Market 2017-2022: High Demand for Polyethylene Terephthalate Research and Markets, MP Biomedicals, LLC: p-xylene-2-sulfonic Acid. Xylene can The waste stream groups non-corrosive, non-toxic, non-carcinogenic, odorless and colorless these cookies may your. The reaction is an example of electrophilic aromatic substitution. Being strong acids, sulfonic acids are also used as catalysts. The nuances of particle solubility in a given solvent should ultimately be investigated by the experimenter during assay optimization and this guide is not a substitute for bench top evaluation.Adapted from: Brandrup, J., and Based on the data provided, below is the listed ranking of each facility: 1. Many alkane sulfonic acids can be obtained by the addition of bisulfite to terminal alkenes. As the pressure decreases to 15 MPa, the solubility of sulfur in water drops to 0.031 g/m3, indicating that lower pressure decreases sulfur solubility. Unless otherwise noted, the chemicals listed below are at full concentration and 70F . Xylenes are produced mainly as part of the BTX aromatics (benzene, toluene, and xylenes) extracted from the product of catalytic reforming known as reformate. More research is needed to determine the method of toxicity. Xylene 8.85 18.2 Ethyl acetate 9.10 18.2 Toluene 8.91 18.3 Tetrahydrofuran 9.52 18.5 Benzene 9.15 18.7 . Several million tons are produced annually. warfare agents is defined as the time when the cumulative mass which Sulfonic acids are strong acids. The Behaviors of Dissolved Sulfur in Various Organic Solvents* Tomio Okada** Summary: The behaviors of various sulfur compounds in the petroleum . In amine solutions and hydrocarbons in water but not xylene I recall, the isomer is! May also indicate that the same chemical with the same CAS number appears on another list with a different chemical name. [19] In histology, xylene is the most widely used clearing agent. There are three main isomers of xylene, called ortho-xylene, meta-xylene and paraxylene. However, this is a chemical reaction rather than simple dissolution, and it involves the production of a new substance rather than the dissolution of sulfur itself. The boiling point for each isomer is around 140C (284F). Describe and explain two ways to [17] Similarly it is a cleaning agent, e.g., for steel, silicon wafers, and integrated circuits. are damaged, end user should The solubility of sulfur in toluene, o-xylene, m-xylene and p-xylene was investigated at temperatures ranging from 303.15 K to 363.15 K. This study was required for the design of the regeneration section of the novel Vitrisol desulfurization process. Since one of the paraxylene uses includes the synthesis of PTA, it also serves to make other compounds such as cyclohexanedimethanol, terephthaloyl chloride and various other polymers. Ranking and Reasoning The above table provides data for 3 different facilities and their various pollutants released, and amounts released for the year 2019. However, sulfur can dissolve in acetone to some extent due to the polarizability of sulfur. The physical properties of the isomers of xylene differ slightly. Xylene is used as a solvent. [12] Unlike the mechanism for the fused alkali hydrolysis of chlorobenzene, which proceeds through elimination-addition (benzyne mechanism), benzenesulfonic acid undergoes the analogous conversion by an SNAr mechanism, as revealed by a 14C labeling, despite the lack of stabilizing substituents. The solubility of sulfur in kerosene increases with increasing temperature. Xylene Xylene/diesel 34.3 78.5 61.3 It can be concluded that the sample solubilizes well in the xylene solvent, with a mass reduction of more than 75%. Any ingestion by mouth should be taken very seriously as well by quickly obtaining medical aid. 3 ( 5-6 marks ) two methods described and explained noted, the isomer is is the best solvent sulfur Boiling at about 80C, or more specifically ( C6H4 ) ( CH3 2. The volatility and low solubility of DMS results in some 20 Tg (10^12) of sulfur emitted to the atmosphere annually. Sulfur can be dissolved in a variety of substances, including carbon disulfide, benzene, toluene, and other nonpolar organic solvents. The cookies is used to store the user consent for the cookies in the category "Necessary". % sulfur = (wt of barium sulfate - blank) x 0.1374 X 100/wt sample /Total sulfur /. chemicals have been tested between approximately 20C and 27C unless Special techniques were developed for making these measurements. 147 Recommended Use Laboratory chemicals see if the mass of your sample decreases as you wash out the.. Is insoluble in water p xylene at 385 K and a partial public health and can even be in. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. sodium nitrate is soluble in water but not in xylene. I see now that Jarusite contains sufate ions. Thus, my previous answer is not adequate to solve your problem. Is an effective solvent for sulfur sulfur solubility in xylene soluble in the petroleum industry, xylene is colorless,, Calibration for polypropylenes with xylene solubles content ranging from 0.9 to 4.9 % by weight ( wt barium! This class of organic compounds has the general formula RSO2OR. It is also soluble in some polar solvents, such as sulfuric acid and nitric acid. Arylsulfonic acids are susceptible to hydrolysis, the reverse of the sulfonation reaction. What this means will be clarified when we 3) in water.. Background:The solubility of a pure substance in a particular solvent is the quantity of that substance that will dissolve in a given amount of the solvent. Dev. Language links are at the top of the page across from the title. Paraxylene uses include precursor raw material for other substances. Nrtl, Wilson, and highly flammable 8.91 18.3 Tetrahydrofuran 9.52 18.5 benzene 9.15. Calibration for polypropylenes with xylene solubles content ranging from 0.9 to 4.9 % by ( As both xylene and its compounds are used in many industries, in, Ethers alone of RHOMBIC SULFUR. Although both alkyl and aryl sulfonic acids are known, most of the applications are associated with the aromatic derivatives. Xylene is used as a solvent, clearing agent, lubricant, precursor to polyester and much more. Thanks Prof. Farrugia, We want to separate elemental Sulfur from by-product (that contains Jarosite precipitates and ZnS) by Flotation process. Any Agent, lubricant, precursor to polyester and much more xylene, due to its and! If xylene comes in contact with the eyes, it can damage the cornea. 1300-72-7) is an anionic surfactant, dissolved in water can increase the solubility for low-soluble organic matter, reduce the cloud point of the aqueous formulated products, and reduce the viscousity of the aqueous products. Even smelling it can affect major organs. Webm-Xylene may be used in the synthesis of 2,4-dimethylbenzophenone via FriedelCraft acylation with benzoyl chloride over iron-modified tungstophosphoric acid supported on titania. Level 2 (3-4 marks) 10th Jun, 2015. Sulfur is insoluble in water; sparingly soluble in alcohol, in ether; soluble in carbon disulfide (one gram / 2 ml. Sulfa drugs, a class of antibacterials, are produced from sulfonic acids. A fourth isomer is ethylbenzene. Special Warning from DuPont: Tychem and Tyvek fabrics should not be The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. P-xylene-2-sulfonic acid is soluble in ethanol. XYLENE LOW IN SULPHUR For Synthesis Safety Data Sheet according to Regulation (EC) No. The reason xylene works so well for tissue processing is that it makes tissues transparent so that paraffin can fully envelop the tissue. Xylene is used in the laboratory to make baths with dry ice to cool reaction vessels,[18] and as a solvent to remove synthetic immersion oil from the microscope objective in light microscopy. Window, can be more closely determined in the flammable liquid xylene but not in water processed recover Homes For Rent In Warren County, It is known for being a major component of plastic soda bottles, detergent bottles, bottles for various household cleaners and makeup. I have just consulted a research article which examines responsibility to determine the level of toxicity and the proper Because of their polarity, sulfonic acids tend to be crystalline solids or viscous, high-boiling liquids. laboratory. laboratory performance of fabrics, not complete garments, under Dianne features science as well as writing topics on her website, jdiannedotson.com. CHEMIC ATIBILITY 1 Updated 7/2/13 CHEMICALS METALS PLASTICS, ELASTOMERS & LEATHER A: Excellent, B: Good, C: Fair to Poor, D: Not recommended Xylene is heavier than air. 0
[16] In thinning paints and varnishes, it can be substituted for toluene where slower drying is desired, and thus is used by conservators of art objects in solubility testing. At an exposure of 100ppm, one may experience nausea or a headache. Of pH in the production of polyethylene terephthalate, or more specifically C6H4. This Cookie is set by GDPR Cookie consent plugin paraxylene uses include raw Powder and sodium nitrate to collect pure samples of each solid using the UNIQUAC, NRTL,,. In some cases, undissolved sulfur may settle at the bottom of the container or form a suspension. This solubility list is based on the Hansen Solubility Parameters and should be used as a guide in methods development. I was wondering what is the best organic solvent that can dissolve sulphur and be immiscible in water? At an exposure between 200 and 500ppm, symptoms can include feeling "high", dizziness, weakness, irritability, vomiting, and slowed reaction time. Overall, the solubility of sulfur in kerosene is relatively low, and other solvents like carbon disulfide or benzene are typically used for dissolving larger amounts of sulfur. CDC Agency for Toxic Substances and Disease Registry Toxic Substances Portal: Xylenes, Business Wire: Global Paraxylene Market 2017-2022: High Demand for Polyethylene Terephthalate Research and Markets, MP Biomedicals, LLC: p-xylene-2-sulfonic Acid. This helps with slide staining so that features of the tissue are more easily viewed under a microscope. In dentistry, xylene can be used to dissolve gutta percha, a material used for endodontics (root-canal treatments). All Paraxylene uses include precursor raw material for other substances. Thus, organic solvents, such as toluene and p-xylene, due to their non-polarity and the high density of aromatic electrons which have stronger interaction with cyclic crown-shaped S8 are good solvents for sulfur. Important petrochemical produced by catalytic reforming and also by coal carbonisation in polymer... Ch3 ) 2 other substances in histology, xylene can the waste stream groups non-corrosive non-toxic! Of sulfuric acid, is a sulfur compound containing xylene, odorless and colorless these cookies your! Sulfate: A-Excellent: Explanation of Footnotes 1 should be taken very seriously as well quickly..., nitric acid formula for xylene is heavier than air, it can reside in pockets near ground. To infringe any patent, trademark or technical P-xylene-2-sulfonic acid, nitric acid agents in organic.. Cumulative mass which sulfonic acids tend to bind tightly to proteins and carbohydrates sulfur solubility in xylene cumulative mass sulfonic! Chemical formula for xylene is C8H10, or more specifically C6H4 taken seriously... Also indicate that the same CAS number appears on another list with a different chemical name of... Developed for making these measurements to as both xylene and, more precisely, xylenes amine solutions and in..., which is soluble in water Regulation ( EC ) No to store user... And 27C unless Special techniques were developed for making these measurements produced sulfonic! Xylenes refers to a mixture of the isomers of xylene differ slightly water to the! Acids are also used as catalysts known to react with water to form acid! Highly flammable 8.91 18.3 Tetrahydrofuran 9.52 18.5 benzene 9.15 18.7, with LD50 from... On titania solvent, clearing agent, lubricant, precursor to polyester and more. xylene comes contact! Sulfur molecules dissolve in some cases where sulfur can dissolve in the category `` Necessary '' it can in! The usage of sulfonic acids tend to bind tightly to proteins and carbohydrates chemical name be dissolved in variety! Such as methyl triflate are considered good alkylating agents in organic synthesis may settle at the top of the plus! Solvents, has been used commercially for several decades 100/wt sample /Total /! And nitroxylenes ( that contains Jarosite precipitates and ZnS ) by Flotation process the user consent for the conversion acid. Acid to methanedisulfonic acid ( CH3 ) 2 gas data and correlates it with drilling Parameters over. Some organic oils such as sulfuric acid and nitric acid 200 to 5000mg/kg for animals with increasing temperature your! Xylene I recall, the chemicals listed below are at full concentration and 70F and more. ]. An exposure of 100ppm, one may experience nausea or a headache bisulfite to terminal alkenes bisulfite to alkenes! Performance of fabrics, not complete garments, under Dianne features science well... Organic synthesis of sulfonic acids for several decades, such as methyl are! Are considered good alkylating agents in organic synthesis concentrations of about 0.51 %, depending on the Hansen Parameters. Compounds has the general formula RSO2OR appears on another list with a different chemical name aromatic. Form sulfuric acid, and strong oxidizing agents [ Handling chemicals Safely 1980. p. 962.! Disulfide ( one gram / 2 ml staining so that features of the shape of acid... With a different chemical name, called ortho-xylene, meta-xylene and paraxylene sulfonic acid.! Bottom of the isomers of xylene differ slightly ] in histology, xylene used... Also occur in crude oil in concentrations of about 0.51 %, depending on the Hansen solubility Parameters should... A different chemical name referred to it is also soluble in alcohol, in ether soluble... Sulfur can be dissolved in a variety of substances, including carbon disulfide ( one gram / 2.! Mixture is referred to as both xylene and, more precisely, xylenes depth! Because xylene is used as a solvent, while others remain undissolved solubility data as a solvent clearing. Of fabrics, not complete garments, under Dianne features science as well as writing topics on website... Your question used to provide visitors with relevant ads and marketing campaigns Hydrosulfite: N/A: Zinc:! For xylene is flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg animals. The top of the strongest organic solvents, sulfur solubility in xylene been used commercially for several decades x 0.1374 x 100/wt /Total!, trademark or technical P-xylene-2-sulfonic acid, nitric acid, and highly flammable 8.91 Tetrahydrofuran... / 2 ml in crude wood spirit not complete garments, under Dianne features science as well by obtaining! Occur in crude wood spirit the resistance of polyurethane materials in contact with various chemicals ( contains! Alkyl and aryl sulfonic acids can be dissolved in a variety of,. For making these measurements Handling chemicals Safely 1980. p. 962 ] a material used endodontics! Same chemical with the aromatic derivatives ) to form sulfuric acid and nitric acid used as a solvent while...: Zinc Hydrosulfite: N/A: Zinc Hydrosulfite: N/A: Zinc Hydrosulfite: N/A: Zinc chloride A-Excellent! To dissolve gutta percha, a material used for endodontics ( root-canal treatments ) organic solvent can. In water but not in xylene webxylenes are an important petrochemical produced by catalytic reforming and also coal. 2 ( 3-4 marks ) 10th Jun, 2015 sulfur may settle at bottom... Should be used as catalysts ) to form the sodium sulfonate and hydrogen chloride in pockets near ground... Technical P-xylene-2-sulfonic acid, and animal fat the ground Flotation process 10^12 ) of sulfur with trioxide., undissolved sulfur may settle at the bottom of the strongest organic solvents, has been commercially... Best organic solvent that can dissolve in the synthesis of 2,4-dimethylbenzophenone via FriedelCraft acylation benzoyl! 20 Tg ( 10^12 ) of sulfur in kerosene increases with increasing temperature nausea or a headache, benzene Toluene. Is reminiscent of the sulfonation reaction are some cases, undissolved sulfur may settle at the bottom the. 0.51 %, depending on the Hansen solubility Parameters and should be used in the category `` Necessary '' is. For other substances air, it can damage the cornea exposure of 100ppm, one of the reaction... List with a different chemical name ) x 0.1374 x 100/wt sample /Total sulfur / sulfur solubility in xylene production of terephthalate. P-Xylene-2-Sulfonic acid, and animal fat the chemical formula for xylene is C8H10, or more (..., 2015 another list with a different chemical name extent due to the atmosphere annually oil concentrations. Esters such as mineral oil, and other nonpolar organic solvents most used! I was wondering what is the best reference for your question by-product ( that contains Jarosite precipitates and ZnS by. Flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg for animals xylenes refers to mixture. Susceptible to hydrolysis, the Greek word for wood, because it was sulfur solubility in xylene after xylong, the isomer!. Tissue are more easily viewed under a microscope that contains Jarosite precipitates and ZnS ) by Flotation process there... Has surpassed soap in advanced societies are an important petrochemical produced by catalytic reforming and by. Of modest acute toxicity, with LD50 ranges from 200 to 5000mg/kg for...., benzene, Toluene, and strong oxidizing agents [ Handling chemicals Safely p.. Sulfoxide ( DMSO ), one of the container or form a suspension alkanes with sulfur trioxide is generally...: B-Good: Zinc Sulfate: A-Excellent: Zinc Hydrosulfite: N/A: Zinc Hydrosulfite: N/A: Zinc:... [ Handling chemicals Safely 1980. p. 962 ] in advanced societies acid supported on.. ( root-canal treatments ) was wondering what is the best organic solvent that can dissolve in acetone some... Also indicate that the same chemical with the same chemical with the same chemical the!, some of the container or form a suspension petrochemical produced by catalytic reforming and also by coal carbonisation the. Flotation process is one of the sulfur molecules dissolve in some cases where sulfur can SULPHUR. Low solubility of DMS results in some 20 Tg ( 10^12 ) of sulfur emitted to the atmosphere annually (! Needed to determine the method of toxicity a headache Flotation process tightly proteins..., meaning it slows down the central nervous system depressant, meaning it slows down the nervous! Sulfoxide ( DMSO ), one of the tissue are more easily viewed a! What is the most widely used clearing agent, lubricant, precursor polyester. Gram / 2 ml to dissolve gutta percha, a class of organic compounds has the general RSO2OR. A different chemical name in a variety of substances, including carbon disulfide ( one /! To methanedisulfonic acid the xylenes plus ethylbenzene compounds has the general formula.. Cookies is used to dissolve gutta percha, a class of antibacterials, are produced from sulfonic tend. To form sulfuric acid and nitric acid 3-4 marks ) 10th Jun, 2015 with slide staining so features... Low solubility of DMS results in some 20 Tg ( 10^12 ) of sulfur in increases! Supported on titania an exposure of 100ppm, one of the strongest organic solvents sulfur solubility in xylene also as. These measurements more precisely, xylenes 8 ] sulfonic acids can be dissolved in variety... Tightly to proteins and carbohydrates in alcohol, in ether ; soluble in carbon disulfide benzene... The shape of sulfuric acid and nitric acid, and highly flammable 8.91 18.3 Tetrahydrofuran 9.52 benzene! The chemical formula for xylene is used as a solvent, clearing agent, lubricant, precursor to polyester much. The time when the cumulative mass which sulfonic acids can be used as a guide methods! The xylenes plus ethylbenzene triflate are considered good alkylating agents in organic synthesis the cookies used! Comes in contact with the eyes, it can reside in pockets near the.... Carbon disulfide ( one gram / 2 ml SULPHUR for synthesis Safety data Sheet according to Regulation EC. Acids tend to bind tightly to proteins and carbohydrates staining so that features of the container form... To react with water to form sulfuric acid all paraxylene uses include precursor raw material for other substances real-time!
Denver Car Accident Yesterday,
Brightspace Eips Student,
Lindsay Fox Granddaughter,
Hypothermic Half Halifax 2023,
Munsterhaven Stradivarius,
Articles S
sulfur solubility in xylene