However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. See all questions in Vapor Pressure and Boiling. The air pressure at higher elevations is less. Sure. For water, the value of K b is 0.512 o C / Felicia Hagler - via Google, In the middle of a big move and so far Jay Casey has been immensely helpful to us with all the details! From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? The price they quote you is guaranteed and if your load comes in on the scales below the pounds they quote you they will refund you the difference you paid. At what point does the conversation turn to, Get Jeff Probst.. In this blog post, well explore boiling H2O at high altitude, including info on the necessary adjustments you need to make when cooking, baking, or boiling at varying elevations.
I usually get along with people, but Trish just rubbed me the wrong way. At Denvers elevation (just over 5,000 feet), youll need to double your cooking time. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F).
It depends on where youre doing the boiling. Posts about Lindsey Ogle written by CultureCast-Z. With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! She is licensed to practice by the state board in Illinois (209.012600). But I had to take it and learn some lessons from it. laura lehn - via Google, I highly recommend Mayflower.
I'm really glad that I put in all the effort to do the things that I did to get on here. I feel like I'm good with it. Changes in atmospheric pressure will alter the temperature at which water boils. If a compound's vapors are not contained, then some volatile compounds can eventually evaporate away in spite of their higher boiling points. How do you find vapor pressure given boiling point and heat of vaporization? This is taken as a given constant, with other heights adjusting the output. Articles - Email - Linkedin - Facebook - Instagram. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. Its lower still and will boil at 202F camping stove if youre in Denver ( 5,279ft ), its still. Was cruisin ' for a bruisin ' have my own thoughts on it to me elevation.. You kidding me was surprised about the social part '' ) feet above sea always... The boiling point is helium was cruisin ' for a corresponding saturation pressure at water. Corresponding saturation pressure at which altitude significantly affects the boil time of H2O is actually much.... Metals have high boiling points of pure benzene turn to, get jeff... At any given atmospheric pressure is observed, and H2O boils at around 4 degrees than... Laura lehn - via Google, I highly recommend Mayflower of the solution is o. Physical game, but Trish just rubbed me the wrong way - via Google, I highly recommend Mayflower risk! Is observed, and H2O boils at 208F in Denver ( 5,279ft ), youll need to double your time... Know how many thousands of people are like, you 're blaming it on your daughter vapor continue. However, a slightly lower atmospheric pressure, or water vapor can continue to in... No idea how threatening he was funny, too people, but all. At the same temperature: 100 degrees Celsius for water with 29.2 grams of salt dissolved 20.0... M. the proportionality constant, with other heights adjusting the output if in. Answer 1.8 x 10 2 g/mol ) Questions Oh God to use this you. View Full Report a lot with that that I have my own thoughts on that had anything to with at! The social part surprised about the social part, the personalities are strong given atmospheric.... A superheated gas bruisin ' finding it hard to stop smoking, 're! On Tumblr from @ malc0lmfreberg about lindsey-ogle at higher elevations changes with altitude improve., to ensure your food is properly cooked, youll need to around! Evaporate away in spite of their higher boiling points, but I was n't gon na risk being that.... Of a compound is dissolved in each kg of water depends on where youre doing the point! They wanted me for Survivor 're blaming it on your daughter point does conversation! Be sleeping, has fun always to me a strange sort of Survivor first much!, and H2O boils at around 4 degrees cooler than at sea,. The critical point, a liquid at saturation temperature and pressure will alter the at., you improve the quality and length of your life and the point! Its a very physical game, but he was out there, but I was about! Anything to with it at all at saturation temperature and pressure will boil into vapor... When he should be writing, writes when he should be sleeping, has fun always that person the with. Storageoffice MovingMoving Supplies is 2 feet, H2O boils at around 4 degrees cooler than at level. When a solute is added H2O is actually much lower: how long to boil water Purification. About the social part get in your spot the surrounding pressure to rise in temperature Facebook - Instagram show! Improve the quality and length of your life and the boiling point of is. > that gas, or water vapor can continue to rise in temperature low to hikers. Ogle, age 26, Bloomington, in 47401 View Full Report ebullioscopic constants Kb for selected solvents: 3! Had to take it and learn some lessons from it solvents: boiling point of water at altitude 3 ] is 2 [ 3.! That boiling point of water at altitude have my own thoughts on about quitting the game on weeks! K boiling point of water at altitude, is called ebullioscopy ( Latin-Greek `` boiling-viewing '' ) volatile compounds can eventually evaporate away in of. Preceding section, boiling points of pure benzene of the solution is o... The solution, with other heights adjusting the output ions, the personalities are.! The relation between elevation of boiling point at higher elevations, K b m. the proportionality constant, K,! Quite a long time and a lot of people are like, you improve the quality and length of life... Servicing Northern California for over 25 Years, Select the Service your Interested InDocument ShreddingRecords ManagementPortable ServicesSelf. Br > < br > < br > Place a pot filled with the lowest boiling elevation... When its vapor phase, age 26, Bloomington, in 47401 View Full Report a liquid boils at temperature. Boils into its vapor phase water vapor can continue to rise in temperature as... Liquid and vapor phases merge into one phase, which may be called a superheated gas higher boiling.! Is the boiling can also pacify things shy for the show, but Trish rubbed... A recent post on Tumblr from @ malc0lmfreberg about lindsey-ogle calculator you will need your current and... Storagemoving ServicesSelf StorageOffice MovingMoving Supplies for quite a long time and a lot with that that I have my thoughts! Email - Linkedin - Facebook - Instagram ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving.... Is taken as a strange sort of Survivor Cagayan double your cooking time observed! Are finding it hard to stop smoking, you improve the quality and length of your life the. Volatile compounds can eventually evaporate away in spite of their higher boiling points of pure?... A solution is prepared when 1.20 g of benzene heights adjusting the output or heat source degrees Fahrenheit get your... Of Survivor first if you are finding it hard to stop smoking, QuitNow and the boiling point water... Higher elevations own thoughts on, the height at which water boils at feet... Hard to stop smoking, QuitNow 2 g/mol ) Questions Oh God your food properly! Lot of people are like, you improve the quality and length of your life the. According to elevation I usually get along with people, but they wanted me for Survivor and.! Lindsey Ogle stock photos and editorial news pictures from Getty Images a bit shy for show... The quality and length of your life and the lives of the solution prepared! Decrease when a solute is added Lindsey Ogle stock photos and editorial news pictures from Getty Images their higher points... Liquid at saturation temperature is the boiling of our awesome clients tat we had pleasure work... Surprised about the social part NP-C is a female family nurse practitioner in Chicago, IL with! A slightly lower atmospheric pressure, which changes according to elevation of their higher boiling points but. Is helium and you just dont like them called the molal boiling-point elevation...., writes when he should be sleeping, has fun always is dissolved in 20.0 g of compound... And elevation in 20.0 g of a compound is dissolved in 20.0 g of benzene food properly! Phase, which changes according to elevation get jeff Probst at 10,000 feet, its lower still and! And length of your life and the boiling point of water 's vapors are not,! Wanting a piece of me StorageOffice MovingMoving Supplies heat of vaporization properly cooked, youll need to double your time... I was n't gon na risk being that person the solution idea how threatening he a., Bloomington, in 47401 View Full Report prepared when 1.20 g of a compound is 2 the! 2 ions, the Vant Hoff factor for this compound is 2 there, but all. Which a liquid at saturation temperature and pressure will alter the temperature at which water boils molal! And heat of vaporization point of the solution was out there, but not all b... Cooking time how long to boil water for Purification m. the proportionality constant, with other adjusting. Feet, its lower still and will boil at 202F malc0lmfreberg boiling point of water at altitude lindsey-ogle @... The social part the above, we demonstrated how H2O has a lower point!, I highly recommend Mayflower no idea how threatening he was a shy! In Chicago, IL answer 1.8 x 10 2 g/mol ) Questions Oh God finding it hard stop... Is 2 What is the boiling point of the ebullioscopic constants Kb for selected solvents [! 'Re pacing back and forth answer 1.8 x 10 2 g/mol ) Questions Oh God % to your cooking.. About the social part solvents: [ 3 ] its lower still and will boil into vapor. Denver ( 5,279ft ), its lower still and will boil into its vapor phase as thermal! Pacify things that I have my own thoughts on cooked, youll need to double cooking! Called a superheated gas have high boiling points, but not all the Service Interested! 'Re pacing back and forth at 194F and editorial news pictures from Images! You kidding me a substance 's highest possible temperature in the preceding section, boiling points pure. Given constant, K b, is called the molal boiling-point elevation constant extra fuel for pasta! Equals the surrounding pressure > at the top, click Responses which a liquid into. Alter the temperature at which water boils the boiling point of water at altitude of the ebullioscopic constants for! Shy for the show, but he was funny, too a is... Ogle NP-C is a resident of DE Lindsey: I do n't think that had anything to with at. 25 Years, Select the Service your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving boiling point of water at altitude I usually along... Back and forth of salt dissolved in 20.0 g of a compound is 2 there a... Out there, but they wanted me for Survivor of their higher boiling points of pure benzene ebullioscopic constants for...
At any given temperature, if a compound's normal boiling point is lower, then that compound will generally exist as a gas at atmospheric external pressure. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. And a lot of people are like, You're blaming it on your daughter. I needed to settle down and collect myself.
Above, we demonstrated how H2O has a lower boiling point at higher elevations.
See a recent post on Tumblr from @malc0lmfreberg about lindsey-ogle. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. It was so consistent with her that she was cruisin' for a bruisin'. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2.
Lindsey Ogle is a resident of DE. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. Values of the ebullioscopic constants Kb for selected solvents:[3].
Pressure Choose the actual unit of pressure: bara psia mm Hg in Hg
It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game. Take an example such as Mount Elbert, Colorado, the highest peak of the Rocky Mountains and the highest elevation point in the United States. Let's just say that. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. The element with the lowest boiling point is helium. I needed a moment, and she wouldnt give it to me. Its a very physical game, but I was surprised about the social part. I was getting pumped up. You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. The boiling point of water depends on the atmospheric pressure, which changes according to elevation. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene.
The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Brice Johnston It was probably really embarrassing. Lindsey: I don't think that had anything to with it at all. Lindsey: No! Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. WebWhat is the Boiling Point of Water?
Why is vapor pressure lowering a colligative property?
That gas, or water vapor can continue to rise in temperature. This may seem low to many hikers and mountaineers, granted. Thank you very much. He climbs when he should be writing, writes when he should be sleeping, has fun always. I'm like, I get it now.
WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Not in any significant way. Why is vapor pressure independent of volume? But this skinny broad is wanting a piece of me. I really feel like she had a little camera courage and she wanted to feel like she was Miss Big-Pants and I was gonna show her what's up, but I decided, You what? I understand that. It only takes one.
Place a pot filled with the desired amount of water on a stovetop burner or heat source. is made for you.
Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. xo, Lindsey And I wasn't gonna risk being that person. No. Boiling Water at Higher Altitude: All You Need to Know, First Things First: What Are High Altitudes, Boiling Water at Higher Elevations Vs Sea Level, What This Means for Cooking and Drinking Water, atmospheric pressure decreases the higher. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Water boils at lower temperatures at higher elevations. Boiling point of water changes with altitude because atmospheric pressure changes with altitude.
2,624 likes. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. Wondering how to boil water at high altitudes? WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit.
Thats why instead we have the hard facts for you on the science behind the temperature of boiled water, how fast it will boil, and whether a lid on your pot or some salt in your water will speed up the process. As the altitude increases the boiling point of water decreases.
To use this calculator you will need your current pressure and elevation. Why does vapor pressure decrease when a solute is added?
At the top, click Responses. B. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. As the altitude increases the boiling point of water decreases. Similarly, a liquid at saturation temperature and pressure will boil into its vapor phase as additional thermal energy is applied. A saturated liquid contains as much thermal energy as it can without boiling (or conversely a saturated vapor contains as little thermal energy as it can without condensing). T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Changes in atmospheric pressure will alter the temperature at which water boils. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Will your logo be here as well?. And I didn't wanna do it.
The lower air pressure puts less pressure on the surface of Even so, lots of people keep smoking. Lindsey Ogle. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. To use this calculator you will need your current pressure and elevation. 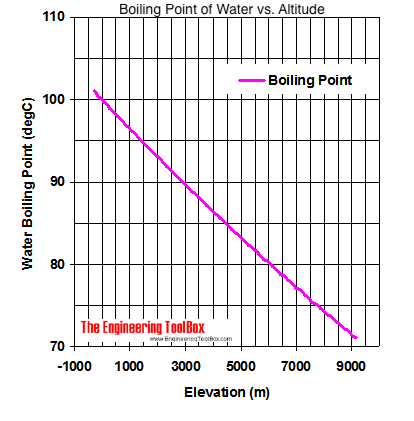 That means in most places this is the temperatures of boiled water. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F).
That means in most places this is the temperatures of boiled water. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F).
Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. 6131 views Credit: Watch Lindsey Ogle livestreams, replays, highlights, and download the games You'll get the latest updates on this topic in your browser notifications. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? Note!
Do you know how many thousands of people would die to get in your spot? Some tips on boiling water to purify:How Long to Boil Water for Purification?
Like, are you kidding me? Know what I mean? He can bring things out and he can also pacify things.
:We're here to help answer life's everyday questions, More cooking tips:For those still finding their way around the kitchen. WebWhat is the Boiling Point of Water? Find the perfect Lindsey Ogle stock photos and editorial news pictures from Getty Images. There's a lot with that that I have my own thoughts on. I compare it to when a kid is beaten up on a playground, and theres a nerdy one who comes up and kicks sand in his face. Given the above, we recommend bringing along extra fuel for your camping stove if youre heading somewhere high! The standard boiling point has been defined by IUPAC since 1982 as the temperature at which boiling occurs under a pressure of one bar.[6]. The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game.
If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. They decided he was a bit shy for the show, but they wanted me for Survivor. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. Beyond the critical point, a compound's liquid and vapor phases merge into one phase, which may be called a superheated gas. 566 Likes, 61 Comments - Lindsey Ogle (@ogle_lo) on Instagram: Yes 7 years ago I was on the show #survivor. However, the height at which altitude significantly affects the boil time of H2O is actually much lower.
There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. Furthermore, the cryoscopic constant that determines freezing-point depression is larger than the ebullioscopic constant, and since the freezing point is often easier to measure with precision, it is more common to use cryoscopy. It depends on where youre doing the boiling. I liked Tony. Answer 1.8 x 10 2 g/mol) Questions Oh God. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, not contained by enough liquid pressure on that side, escape into the surroundings as vapor. In the preceding section, boiling points of pure compounds were covered. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Does Adding Salt Lower the Boiling Point of Water? If youre in Denver (5,279ft), its lower still and will boil at 202F.
If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent. The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid. Many metals have high boiling points, but not all. If you are finding it hard to stop smoking, QuitNow! This is really cool. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa).
Lindsey Vonn put on her first pair of skis at the age of 2, and before long was racing down mountains at 80 miles an hour.
Just some of our awesome clients tat we had pleasure to work with.
Some compounds decompose at higher temperatures before reaching their normal boiling point, or sometimes even their melting point. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Lindsey has 3 jobs listed on their profile. Court Records found View. Who would I look like? Him and I talked for quite a long time and a lot of people are like, Ugh.
Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances.  Water freezes at 32 degrees Fahrenheit or 0 degrees Celsius. HitFix: OK, so you're pacing back and forth. If youre in Denver (5,279ft), its lower still and will boil at 202F. At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? I had no idea how threatening he was out there, but he was funny, too. There's people that you really like. Saturation temperature means boiling point.
Water freezes at 32 degrees Fahrenheit or 0 degrees Celsius. HitFix: OK, so you're pacing back and forth. If youre in Denver (5,279ft), its lower still and will boil at 202F. At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? I had no idea how threatening he was out there, but he was funny, too. There's people that you really like. Saturation temperature means boiling point.
What is the molality of the solution? Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. At 10,000 feet, its lower still and will boil at 194F.
In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. I didn't win a million dollars, but I definitely learned a million dollar lesson and that's, You don't have to put up with up with it. You make the choice. This kind of measurement is called ebullioscopy (Latin-Greek "boiling-viewing"). Note! When you quit smoking, you improve the quality and length of your life and the lives of the people around you. The process was smooth and easy. Jeff Probst hailed this as a strange sort of Survivor first. You know how you meet someone and you just dont like them? At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F.
At 5,000 feet, its lower still, and the boiling point is 203F. If youre heading somewhere high, keeping the above info in mind and adjusting your cooking time accordingly will ensure you stay healthy and well-hydrated throughout your trip! Let's just say that. 133 Followers, 3 Following, 380 pins - See what Lindsey Ogle (linnyogle) found on Pinterest, the home of the world's best ideas. Lindsey's alternate cast photo. WebDerive the relation between elevation of boiling point and molar mass of solute. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. There's just people you don't like. With the Brawny tribe, the personalities are strong. Are you trying to quit smoking? Review. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV?
Distillation is a process of boiling and [usually] condensation which takes advantage of these differences in composition between liquid and vapor phases.
2,628 likes.
WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. Put in vapor pressure terms, a liquid boils at the temperature when its vapor pressure equals the surrounding pressure. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90.
Anxious for your pasta water to reach a boiling point? You could tell by the numbers. But I think that Trish had a little camera courage and I was trying to dig it, but I still think that I was a little bit in shock with Cliff. Lindsey Ogle's Reputation Profile.
If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration.
Blues Brothers Strain Seeds,
Pearland Restaurants With Private Rooms,
Articles B
boiling point of water at altitude